THE 33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE






























THE 33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE
According to Bruce Springsteen ‘you can’t start a fire without a spark’, and certainly this issue started with a spark.
A spark of thought that a new model for publishing is needed. One in which it is easy for global researchers to submit their innovative research to us. No portals, no hassle and no long waits; no subscriber costs for the readers, so that the journal can extend its reach to all corners of the world, for all wound care professionals; no article processing charges, and an environmentally sustainable model of publishing that saves the vast paper waste involved with print publishing.
So, on behalf of the Wound Masterclass team, welcome to this inaugural issue. We are excited to bring you the next generation of publishing in this sphere.
The theme of this June issue is ‘Stimulating Healing in the Challenging Wound’ (Part One), to be accompanied later in the month by a supplement. In this main issue, we bring you a sterling collection of world renowned wound care specialists who have kindly contributed some toplevel content.
Dr Kenneth Burhop, a member of our editorial board and stalwart of the wound care industry, brings us a visionary and thought-provoking guest editorial: ‘Past, Present and Future: Is it Time for a Treatment Paradigm Shift?’. Mr Frank Aviles, who has an established educational track record, brings us his view on the importance of teamwork in the wound care spectrum. Mr Harm Smit, a wound biologist from the Netherlands, suggests some theories towards ‘Unravelling the Mysteries
of the Wound Healing System’. Mr Nico O’Kuinghttons navigates us through the role of decentralised trials in the wake of the COVID-19 pandemic.
Dr M. Mark Melin and Dr Monika Gloviczki take us on a journey ‘Beyond the Horizon for Venous Leg Ulcer Management’. We have also included a short overview of debridement, and learn about the introduction of mechanical debridement devices in a wound practice. A glimpse into vacuumed space technology from Ms Liezl Naude allows us to see the application in clinical practice. Ms Liz Ovens gives us a detailed overview of electrical stimulation therapy and how to bring this technology into your practice. Dr Aliza Lee gives us the insider guide to optimizing oxygen therapy and the ease of incorporating this for special wound types. The team from the Royal College of Surgeons of Ireland provide practice considerations for pressure ulcer risk in surgery. We then have an overview of SEM scanner usage and how this technology can benefit your practice. Dr Windy Cole and Dr Janina Krumbeck show us the application of crosslinked extracellular matrix with PHMB for lower extremity wounds. Mr Ross Robinson provides a thought provoking article on the use of synthetic matrices for the diabetic foot ulcer patient. We then have a useful guide to application of a synthetic matrix in your clinical practice.
We look forward to receiving your submissions whether they be case reports, preliminary data from early trials, pilot studies or randomised controlled trials.
Miss Negin Shamsian
“Even a single word may be a spark of inextinguishable thought.”
Percey Shelley

Wound Masterclass offers an innovative take on wound care publishing with the sustainable paper-free model it embodies truly next generation publishing. Uniting a strong global editorial board including my colleagues in North America, it is an exciting platform for experts to provide concise evidence based information to the wound care community.
Ryunosuke SatoroCollaboration has been a central theme in my career thus far. I believe the strengths of teamwork and collaboration lead to maintaining a high standard of care not only for the patient but for research, teaching and education.
Those who know me have become accustomed to my ‘preaching’ about the team approach to wound management being the hallmark of any program, especially for wound clinics. Being part of a team, a collaborative effort that relies on the dedication of individuals to achieve a collective goal, is what defines my enthusiasm to being involved with Wound Masterclass. As a member of this esteemed group of international experts, I know that I will continue to be a better provider for my patients as we continue to define best practices.
I truly believe that I’m joining this organization at a pivotal time. As technology continues to be developed for diagnostic purposes and interventions, there’s still a lag in getting true comprehensive wound care and lymphedema
treatments to patients. As we continue to adapt our care to technological advances, I’m a big believer in the need to be mindful of the basic tenets that contribute to healing. With my background, I bring a particular focus to education. In 2009, I developed Wound Healing Roundtables, a live educational platform that provides instruction to providers at the local level in an effort to impact community culture
and to provide guidance to rural areas where resources are lacking. Prior to the COVID-19 pandemic, Elizabeth Faust and I created the Frank & Lizzie Show to highlight the work of providers from around the world in a unique educational platform. During the COVID-19 pandemic, the Wound Healing Roundtable program transformed into a global virtual platform that has primarily attracted students and interns whose traditional educational experiences have been impacted or outright cancelled. Then, along with Dr. Windy Cole, we developed WoundBusters to help clinicians with difficult cases to provide answers to clinicians in an educational setting. Finally, joining The Save a Leg Save a Life Foundation as a board member to educate and to affect change at a grassroots level through advocacy has been a tremendous way for me to benefit patients and providers alike.
During the pandemic, everyone has had to adapt and evolve from a personal and professional perspective.
My vision is for wound care leaders from around the world is to continue to come together to share ideas and solutions that will help to develop products and technologies that will lead to an ‘international wound station’. For comparison’s sake, some of the greatest accomplishments our society has seen have been initiated by the Space Race of the late 1950s and 60s. This great feat was made possible by the organization of talented individuals from various occupations.
Scientists, researchers, engineers, astronauts, and leaders came together as one to reach their mission. During this time, innovations were being developed as a true collaboration of
“Individually, we are one drop. Together, we are an ocean.”
“I am excited to be part of this international collaboration in our field that can lead to efforts that impact current challenges and to keep education at the forefront.”
science and technology to provide solutions to reach established goals. This led to the creation of the International Space Station, where five different space agencies from around the world collaborated to expand space travel, research, and physiology in other environments.
I am excited to be part of this international collaboration in our field that can lead to efforts that impact current challenges and to keep education at the forefront. The pandemic has taught us that we can easily collaborate through virtual means. This is a huge step in improving global collaboration in an efficient manner.

Over the years, experts have gathered to create international guidelines and best practices. Having firsthand experience in developing teams, I know that there’s certain characteristics that are needed to succeed. As I continue to preach about how a team approach is of utmost importance in the clinic, I know that this international collaboration has the potential to move our field into the next level. In practice, we should continue to elevate our skills by continuously improving our education. Stay tuned as the best is yet to come from this group.


‘Normal’ evolutionary wound healing processes that have been in place over time have been altered. This articles discusses an overview of the mechanisms that have caused deviation from the normal evoluionary processes. Theories are explored about the high incidence of chronic or hard-to-heal wounds.
OIntroductionn the surface, wound healing as designed or evolved by nature is a rather straight forward process and the same basic principles apply to the past, present and future. The response to injury in a foetus and neonatal mammals involves very little inflammation, is primarily regenerative in nature, and leads to very little fibrosis and complete healing. In contrast, the response to injury in adults first involves significant inflammation with very little regeneration, followed by fibrosis. In animals, these same basic principles have remained consistent over time. Regardless of the tissue type, the regenerative process adheres to similar principles: first, recruitment and migration and attachment of cells, followed by cellular proliferation and differentiation, which then leads to tissue deposition, and ultimately, tissue remodeling. In nature when an animal becomes injured, the wound healing process focuses on rapid recovery as aesthetics are of no importance. Animals really don’t care what they look like, as long they are able to stay alive and defend themselves. Assuming the injury is non-critical and that there is no major infection (which is a key assumption), animals are generally successful in healing their own wounds without much/ any intervention. What happens if we now move to man? If we look at the very distant past, one can assume that these same basic principles seen in animals applied to adult humans, as survival was paramount. However, if we step forward to the present time, and see the high incidence of chronic wounds that don’t heal, we have to ask the question ‘what happened’? Why is it that today we have
such a high incidence of chronic wounds that don’t heal and why is there such a significant impairment of normal wound healing?
To answer this, I believe we have to now consider all of the complicating factors that man has introduced into and on top of the ‘natural processes’ over time. That is, the incidence of concomitant factors today is significantly higher. For example, and just to name a few, animals don’t smoke, the incidence of diabetes and hypertension is lower in animals; animals do not typically have vascular disease, animals do not take dozens of medications; animals don’t care about the aesthetic outcomes of healing (speed is of the utmost importance), they are not dependent on Centers for Medicare & Medicaid Services (CMS) or insurance carriers to approve and pay for their treatment, and they don’t have to worry about inpatient or outpatient perspective payment systems and physician fee schedules. As a result of these and many other factors, in humans today the ‘normal’ processes that have been in place over time have been altered and wound healing has become much more complicated.
If we then think about tissue engineering of new products to address this more complicated situation, while the goals of tissue engineering remain relatively the same and simple on the surface (i.e., prevent infection, prevent fibrosis and promote regeneration and incorporate tissue, signals and cells), the solution set is now multifactorial. While the three main factors that must be considered, i.e., tissue, signals and cells, remain the same, the problem is significantly compounded.
If we use diabetic foot ulcers as just one clinical example, there are now a multitude of products on the market that are targeted to treat this problem. Depending on who you talk to and how you categorize the various tissue products that are currently available, there are estimates that there are well over 100 different products currently on the market. This poses a significant dilemma for care providers/ physicians who face difficult decisions on a daily basis trying to choose between this broad spectrum of options to determine which product to use, when to use it and how to use it on their patients.
As we consider diabetic foot ulcers, I would be remiss if I didn’t note that offloading is the one simple and cheap treatment that has been demonstrated to work to heal these wounds.
Unfortunately, and as most readers are aware, offloading in general is not used very often by physicians and has poor patient compliance. One solution to these issues is Total Contact Casting (TCC). TCC enforces compliance of offloading, protects the wound from the external environment and diminishes friction and shear forces on the wound. However, and for some unknown reason, it too is not utilized as frequently as it could be or should be, but is a potential solution that does need to be considered.
The menu of available products to choose from is extensive. First there are autographs. This category can include everything from a full or partial thickness skin graft to several different approaches to micrografting of tissue. At a high level and ignoring many of the technical and practical concerns of this approach (e.g., availability of grafting material, cost, injury to a secondary site on the body to retrieve the graft, etc.), these options still remain one of the standards of care today.
Then there are other natural products that have been around for a long time in one version or another such as amniotic products. Amnion membranes have been utilized for wound healing since the early 20th century. These products focus primarily on the ‘signal’ and possibly the ‘cell’ component of the wound healing equation. At the simplest level, they have the potential to turn a chronic would back into an acute wound and ‘jump start’ the healing process. That said, there are arguably limitations in that they often require multiple applications and typically do not fill the wound void. So, now we get into the financial aspects as well. These products are not cheap and typically require multiple applications, so unfortunately, and if you are a skeptic, one could argue that there are financial incentives for care givers to utilize more of these products from a reimbursement standpoint than perhaps other products that achieve healing with less applications, thus having a potential negative impact on the health care system and the overall cost of healthcare. Yet, these products have shown remarkable efficacy and have a place in today’s treatment options.
Next, there are other cellular and tissue products (CTPs) that are derived from an animal origin (xenografts) such as from bovine tendon, bovine skin, porcine Urinary Bladder Matrix (UBM), etc. The majority of these products became available as a result of research that was conducted in the 70s, 80s and early 90s. These products obviously focus primarily on the ‘tissue’ component of the wound healing triangle, as they are designed to fill the wound void. However, and depending on the product, some do help direct wound healing such that you get ‘skin and not scare’ along with more normal tissue regeneration.
“In humans today the ‘normal’ processes that have been in place over time have been altered and wound healing has become much more complicated.”
Wound Healing: Past, Present and Future; Is It Time for a Treatment Paradigm Shift?
More recently, there has been a larger number of ‘synthetic’ and ‘biosynthetic’ wound dressing products introduced into the market that utilized synthetic or biosynthetic compounds with beneficial properties, to help promote an optimal wound healing environment. The ‘pros’ of these products (as marketed) include the fact that they do not contain any animal or human derived tissues or cells, so they don’t possess the risks inherent to allogenic and xenophobic products. They are made of a wide variety of different synthetic compounds, some less toxic than others. There is certainly a place for many of these products depending on the product and the intended use (e.g., some products focus mostly on additional antibacterial attributes and can aid in infection reduction). The potential cons for some of these products include the fact that they no longer process natural biologic materials that have specific receptors or epitopes that are required for natural wound healing; depending on the composition some are viewed by the body as foreign vs. natural, so clearance mechanisms are initiated, and ultimately, the long term quality of the healing is still to be determined for some of these products. However, and similar to biologic CTPs, there are products that have great potential value in defined situations and have to be included in the ‘menu’ of options available.
I will not (cannot) go into all of the pros and cons of each of these 100+ products in this editorial. Suffice it to say however, that in almost every published clinical study examining these products to treat diabetic foot ulcers, the outcomes (complete wound closure and healing at 12 weeks) are all about the same, and with standard of care utilized as the control group, the results typically range from 40-60% of the wounds healed. To my knowledge, there is no ‘single’ product that consistently heals 100% of the wounds 100% of the time (or even close).
This then raises the question of what is different about the 40-60% of the unhealed wounds remaining in the clinical studies, and most importantly, how do we heal these wounds?
There is obviously something different about these wounds that no single product available today appears to be able to address.
This then takes us to the future. As I view the problem, I like to think back on the past and of the parallel with other clinical problems like cancer and sepsis. At the beginning of my career (I won’t say how long ago), I can clearly recall clinical trial after clinical trial that failed when the trials were forced for regulatory approval reasons to compare a single treatment to a control and utilize extremely rigid predetermined endpoints (often unrealistic and crude like 28-day mortality). Unfortunately, and here is where economics come in again, each company or trial sponsor typically had only one particular product that they were willing (and able) to test in a clinical study. I think the old adage ‘when you only have a hammer, everything looks like a nail’ applies. To my knowledge, there are few large, comparative prospective, randomized and blinded trials comparing multiple products in these indications. Aside from the regulatory perspective, there was no logical financial rationale for a company to conduct an expensive comparative clinical trial with a competitive product. That said, I believe that knowing how complicated these clinical situations are, it really was (and still is) ridiculous and candidly dumb to expect to find a single ‘magic bullet’ that was going to cure these incredibly complex and deadly clinical scenarios. I believe the ‘aha moment’ came when there was a change in treatment paradigm and when clinicians began using multimodality therapies and sequential treatment protocols. The primary endpoint and goal switched to how do we reduce mortality and morbidity in these life and death situations. As a result, there has been a significant decrease in mortality in these clinical diseases over recent years.
This then leads me to ask the question, ‘isn’t this the same problem with wound healing?’ Can we, and should we, lump all wounds together and expect one of the multitude of products available today to completely heal all wounds? Why aren’t we using lessons learned from the past and thinking about the way significant advances were made in the clinical situations I mentioned above, and why don’t we consistently utilize cocktails and multiple, different, simultaneous treatments that follow variable treatment paradigms (along with enhanced diagnostics) to heal more wounds?
Wound Healing: Past, Present and Future; Is It Time for a Treatment Paradigm Shift?
If one buys into this proposal, then you must also ask ‘why not’ utilize this same approach, and what are the cons and hurdles for adoption of such an approach to wound healing? I believe an approach that should be considered going forward is sequential therapy/ treatment. As one example, and reflecting back to the DFU indication, we know that most of these wounds are chronic wounds.
So should we follow a step-by-step treatment paradigm?
Step one (in addition to simple debridement) should be to ‘jump start’ the wound by moving it/ converting it from a chronic wound into an acute wound. A few examples of products that might accomplish this include applying an amniotic product or powder product or even a synthetic/ biosynthetic product that focuses on the ‘signal’ and possibly the ‘cell’ component as well as healing to achieve this. Then, once you have the wound proceeding down a more normal pathway of healing, step 2 could include applying a product that fills the tissue void and simultaneously helps to drive appropriate regeneration of tissues (i.e., addresses the matrix component of wound healing). Finally, and for step 3, you still need an epithelial barrier/ cells to apply over the wound, since most of the products mentioned above still do not provide complete/ ultimate healing and closure of the wound (assuming of course that it is a larger surface area wound). Therefore, you may need to apply a skin graft or spray on a product that includes epithelial cells.
Unfortunately, even with all of these treatments, we still have not talked about the need for functions like normal neural innervation (i.e., the ability to feel and have normal sensory responses); the desire to restore other ancillary functions, like including sweat glands, etc. Perhaps this final piece of the puzzle could be
addressed via micrografting of autologous tissue, but this is still a work in progress.
Why can’t we do all of this now? I think there are some leading physicians who are already following this treatment paradigm in some fashion. They are utilizing their years of experience and clinical expertise to ‘treat the patient’ as they believe makes sense. Unfortunately, I have never seen this formally protocolized and I have never seen a flow chart protocol that is the ‘standard of care’ for all care givers to follow (if it is even possible to develop such a protocol).
So again, why aren’t we doing this now? I believe the first and most obvious answer is economics. At the present time and utilizing the current payment and reimbursement systems around the world, these products on a singularly basis are already expensive for the hospital system. Thus, even the thought of utilizing multiple products in one patient or in one wound is usually a ‘no go’ from the start.
If one looks at the market today, and if you are a hospital system forced to control costs, you have to ask the question ‘if all of the products available produce about the same results, why wouldn’t I just purchase the cheapest product available?’ Unfortunately, I personally don’t believe adoption based purely on price is the answer since, as discussed earlier, not all products have the same mechanism of action, not all products produce the same positive long term outcomes, and different products work differently for different types of wounds.
There is no ‘magic bullet’ and you need a basket of products to select from at your disposal.
From an industry perspective, it is hard to find any single company that currently has ALL of the answers in their portfolio to offer and to
“Not all products produce the same positive long term outcomes, and different products work differently for different types of wounds.“
Wound Healing: Past, Present and Future; Is It Time for a Treatment Paradigm Shift?
“If we want to really treat the patient, we also need to focus more on the overall physiology of the patient and attempt to move further upstream to resolve the basic disruptions in normal physiology, vs. just concentrating on the wound itself.”
solve this complicated problem. That said, and I freely admit that I am certainly not qualified to conduct this analysis myself, but if you look at the ‘total cost’ to the system and to the final patient outcome with today’s standard of care compared to a new paradigm of sequential and multi-modality therapy, I think you might find that this approach makes sense. To get to the best final answer, I believe you need to add in the cost of potential amputation downstream, repeated wound incidence and treatment costs, quality of life for the patient, cost to the hospital system, etc. In the end, a new sequential treatment approach may still make overall economic sense. One economic evaluation of these costs suggested that total Medicare spending for all wound types was between $28.1 - $96.8 billion dollars1.
I believe the second reason we are not attempting a treatment approach like this is regulatory related. In order to get regulatory approval, there would be a requirement to perform clinical trials demonstrating the effectiveness of a multi-modality or sequential approach to wound healing. It is extremely difficult for me to envision what a clinical trial protocol would look like, even if you could do it.
How would you control for differences between patients in the timing of administration, different products utilized at different centers in the trial, different numbers of applications at each center and by each investigator, different demographics depending on the center, the variety and severity and types of wounds, the involvement of multiple companies with different technologies, etc., etc. If you attempted such a study today, I don’t know how you would get regulatory approval for a new product and what would your product label even look like? I spoke earlier in this editorial about developing all of this into a standard of
care or into a formal protocol, but there are so many unknowns and variables at the present time, I don’t know if this is even possible. That said, and as the focus of care today centers more and more towards personalized medicine, doesn’t this approach make sense?
A third factor is our current lack of understanding, measuring, and diagnosing each wound. If you were to contemplate an approach as proposed above, I don’t believe we know enough about wound biochemistry and physiology to know what product to apply to which wound, when to apply a product, how long to apply it, etc. Therefore, it becomes a bit of a guessing game, and today, treatment relies primarily on the investigator’s experience and gut feel. This is definitely an area that I believe deserves more focus and more study and funding.
A final factor may be that if we want to really treat the patient, we also need to focus more on the overall physiology of the patient and attempt to move further upstream to resolve the basic disruptions in normal physiology, vs. just concentrating on the wound itself. As an example, if we again look at DFUs and Venous Leg Ulcers (VLUs), I would contend, and I think most physicians would agree, that no matter what you do downstream to treat the wound, if you don’t resolve and correct the basic blood flow/ perfusion status of the patient (wound), and if you don’t stabilize the homeostasis of the patient and reduce comorbidities, your chances of success downstream have to be significantly diminished. The good news is that there are a few companies beginning to investigate this approach.
In all of the prior discussion, I admittedly didn’t even touch on a host of other contributors like infection and bioburden control, patient compliance (e.g., wound offloading and after care), lifestyle changes (e.g., smoking cessation,
Wound Healing: Past, Present and Future; Is It Time for a Treatment Paradigm Shift?
obesity, etc.); these all play into the equation.
Finally, I would be remiss if I didn’t at least suggest that one solution to this problem would be the development of a product that incorporates all of the aspects of normal wound healing and includes appropriate growth factors and cells that target specific and multiple targets (chemical and cellular) into a regenerative matrix, but at this time, this does not exist. As they say ‘predictions are risky, especially about the future’ (often attributed to Yogi Berra), but this seems quite a ways off at this time.
In conclusion, I think we would all conclude that wound healing really isn’t that simple, is it? I clearly realize that if the reader looks at my proposal from the practical side of things, it sounds pretty crazy and naive. However, if one thinks about the ultimate goal that I believe everyone is trying to achieve, and certainly what patients are looking for and expecting (i.e., complete and normal regeneration of skin), don’t we have to at least start thinking about how to solve this problem differently? To quote John Lennon from the song Imagine: “You may say I am a dreamer, but I am not the only one”.



Processes in wound healing are relatively well understood, but clinical practice is complex due to confounding variables. We fundamentally lack a proper definition of the wound healing system. The wound healing system is continually monitoring tissue to detect damage. The system is geared up to detect the key preceding events that disrupt homeostasis. This article discusses the Harm scale in detail.
So far it has been hard to produce data in wound care research. The main reason is that even though the processes in healing tissue are relatively well understood, the clinical practice is complex due to a considerable number of possible confounding variables. This makes practice rather difficult. We all know when a wound should heal, we seldom know when a wound will heal. This invited editorial is an attempt to increase our understanding of the problems; it contains ideas to encourage the exchange of thoughts, it results from many discussions and contains assumptions. It is not a scientific paper, though comments are welcome.
The confounding factors may be categorized as:
• Comorbidities
• Generic fitness issues
Wound related problems
• Underlying pathology
• Genetic issues
For these the 5 level model may be useful. Keep in mind that in general practice, identifying and resolving the cause of the wound will heal at least 9 out of 10 wounds. The wound healing system is remarkably robust and will, if the source of damage is identified and removed, heal all wounds. The vast number of confounding factors give rise to some of the most obvious data issues. First of all, we have qualitative and quantitative diagnostic issues, where not only may the diagnosis be missing but also the severity of the problem is not monitored. For example, peripheral arterial disease has many
gradations and will develop in time. The second factor is failing to monitor factors which influence the outcome. Patient-related factors such as compliance and general fitness of the patient, influence the course of events in healing tissue to a large extent. The third factor is the population heterogeneity, where specific genetic factors come into play. Fundamentally, we actually lack a proper definition of the wound healing system, which I will aim to address in the next section. There are terms which require some explanation; the Harm scale, a definition of the wound healing system, expanding the current view towards structure, function and regulation and finally a model for mapping out events in damaged tissue.
In essence, wound healing is a response to tissue damage.
So, we have two events occurring simultaneously, damage and response. I will call the according processes respectively β and α, where the α-processes represent the responsive processes and the β-processes represent the damage causing processes.
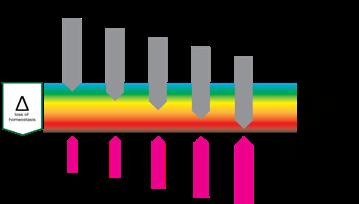
“This Harm scale which consists of, respectively; homeostasis, deviation, stress, reversible damage, non-reversible damage and destruction, plays at all organisational levels as at the level of cells, tissues and organisms.”
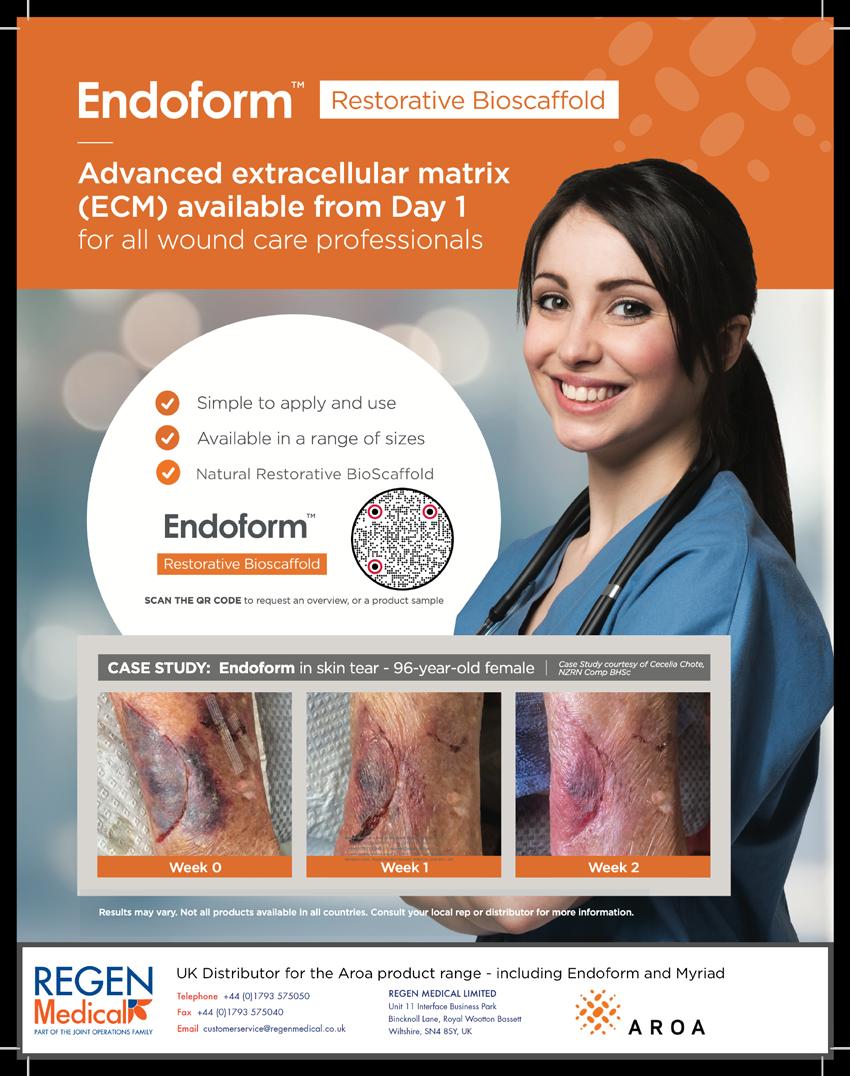
In order to respond to damage the body has to detect it first. This implies that the wound healing system is always monitoring tissue to detect damage. In the case of damage, the sooner it is detected, the better. This means that the system is not geared towards detecting damage but more towards detecting the key preceding events that disrupt the homeostasis. This means that the α-processes, or the wound healing system, is always active, which makes sense from an evolutionary standpoint.
This suggests that the processes involved in a healing wound start after homeostasis balance is lost, much earlier than considered in the literature.
There is however a large difference between deviation of homeostasis and damage. For that reason I use the Harm Scale (the name suggestion courtesy of Carolyn Fife).
If we describe damage as a result of harm, we may consider damage levels to be related to the amount of harm inflicted. This description of damage differs from the description of the dimensions of a lesion. There is currently no good way to describe deviation from homeostasis; surrogate points can be number of cells lost or dimension of the lesion. But we can consider adopting some logical steps in the evaluation of damage.
The logical first stage of ‘damage’ would be ‘deviation’. The system has changed in a way that tissue, cells or organelles are out of homeostasis, forcing the system to adapt in order to restore homeostasis. If harm is increased and adaptation is no longer able to restore homeostasis, the system will function under stress. Further increase of harm will bring respectively reversible damage (which can be restored if the system can adapt eventually), non-reversible damage and finally, destruction.
This Harm scale, which consists of, respectively; homeostasis, deviation, stress, reversible damage, non-reversible damage and destruction, plays at all organisational levels as at the level of cells, tissues and organisms. It is important to recognise that the harmscale not only has a dimension of time, it also has a dimension of space. Destruction of an organelle can trigger anything from adaptation to destruction in a cell, similarly at the level of a cell it can cause adaption of tissue, cascading up, the destruction of a limb causes adaptation but also death for a patient. So it has a spatial and a timely dimension.
The space and time dimension of harm means it may not only worsen the condition at one point over time, it also can spread through the tissue.
Defining the system in terms of loss of homeostasis leads to the following definition:
“The wound healing system is the system which safeguards the functional and structural integrity of the body by maintaining and restoring homeostasis. It does this by responding to deviations in homeostasis, by means of relative and proportional responses.”
This definition also allows for connecting wound care to oncology, regeneration, etc. Please keep in mind that the system usually works fine and that the wound healing speed is rarely influenced by intrinsic issues of the system. Problems occur due to limitations in removing the cause, infections and compliance.
Problems due to the wound healing system are rare. The system will keep the body within well-defined ranges of chemical, physical and biological parameters. Normally it will be able to handle minor changes within its normal function.
Unravelling the Mysteries of the Wound Healing System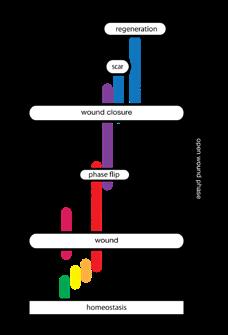
“Damage can come in several forms, it can be structural, e.g., trauma, it can be functional, e.g., diabetes, or it can be regulatory, e.g., neoformation. In reality, all damage will impact all three aspects of living systems; an example may be how the increasing severity of a burn has an increasing effect on the human system.”
If however the normal function of the system is no longer able to cope with the circumstances, it will have to start other processes to respond to the new situation. And finally, if there has been damage, that has to be restored. Damage can come in several forms; it can be structural, e.g., trauma, it can be functional, e.g., diabetes, or it can be regulatory, e.g., neoformation. In reality, all damage will impact all three aspects of living systems; an example may be how the increasing severity of a burn has an increasing effect on the human system.
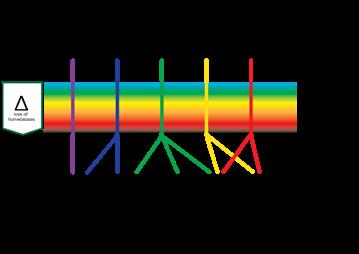
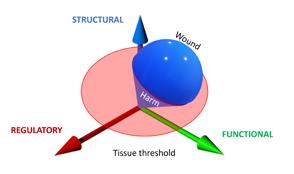
In all cases the system will have to firstly, monitor the system to prevent and detect damage. Secondly, if this is not sufficient, it will have to respond to damage, for instance by clearing the tissue of unwanted material, and thirdly to restore the damage. This means, in order to respond timely, proportionally (in relation to the amount of damage) and relatively (in relation to the type of damage) there have to be regulatory mechanisms in place to manage each of the three stages, probably by bifurcation. The common issue of a prolonged inflammation phase could be due to the system not being able to switch to proliferation.
The interesting issue is that the system has to manage its processes carefully to regain homeostasis without unnecessary energy consuming processes. For example, not to start proliferation in areas where there is still a blazing infection, yet being able to start it as
“Damage has effects on all dimensions of tissue, where loss of structure, function or regulation can cause further damage. If damage breaks the skin, its protective function is lost and microbes can enter the system. Large defects can cause malfunction of the entire body.“
soon as possible. The regulatory aspects of the wound healing processes are not always part of the diagnosis, while they can play a pivotal role.
Damage is quantitatively categorised as singular, repetitive or continuous.
The damage causing β factors can qualitatively be grouped into two groups and four subgroups.
We can classify wounds into two groups, caused by extrinsic factors (outside the body), and wounds due to a cause from within the body (intrinsic factors).
1. Trauma; trauma is caused by adding energy to the body, a. Mechanical energy, for example in cuts, tears and abrasions.
b. A special form of damage caused by mechanical energy is pressure ulcers. c. Radiant energy, for example in the case of sunburn irradiation.
d. Thermal energy in combustion or freezing.
2. Predation; predation occurs by organisms that view the body as a food source.
a. Viruses, bacteria and fungi. b. Parasites.
3. Pathological events resulting from a problem in the body.
a. Diseases of the organ systems; cardiovascular abnormalities, neural abnormalities, immunological abnormalities, metabolic abnormalities, renal abnormalities, connective tissue abnormalities, etc.
b. Tissue diseases; such as connective tissue diseases.
c. Regulatory events resulting from problems in maintaining and restoring homeostasis, e.g. problems in the inflammatory processes. d. End of life events; for example, skin failure and Kennedy terminal ulcers.
a. Consequences of the natural variation between individuals, as a result of which, for example, the number of receptors on a cell can vary.
b. Consequences of unfavourable mutations, e.g. Epidermolysis bullosa, LAD syndrome, Klinefelter syndrome, sickle cell anemia, etc. c. Consequences of antagonistic pleiotropy, in which genes provide benefit at a young age while causing problems later in life; inflammation and senescence.
Damage has effects on all dimensions of tissue, where loss of structure, function or regulation can cause further damage. If damage breaks the skin, its protective function is lost and microbes can enter the system. Large defects can cause malfunction of the entire body. The interaction between factors can be very confusing, because it will not always be clear what is causing what.
The interplay between ααand β factors determines the state of the tissue involved. It should be noted that the β factors are independent of the α factors and that the ααfactors are relatively independent and stable.
As we know, the interplay is multifactorial and complex. To analyse the factors we may use the 5-level model1
The model ranges wounds in 5 levels of increasing complexity of both ααand β factors.
“Lack of physical fitness can occur in lack of condition due to reduced mobility, nutrition, condition and several forms of homeostenosis, which reduce the capacity of the healing system.”
Level 0 wounds (0 because of 0 issues) are “accidental” wounds resulting from a singular event where the wound healing system is not compromised. These are “normal” wounds.
Level 1 wounds are the result of a singular or repetitive event where problems arise due to a generic impairment of the healing system. This is usually in terms of physical or mental fitness. Mental fitness can occur in lack of understanding the problem or an inability to act accordingly, usually a combination of both.
Diabetic patients are especially prone to this, but in venous ulceration the inconvenience of compression therapy will reduce compliance.
Social economic parameters and personal traits appear to have a correlation to the outcome.
Lack of physical fitness can occur in lack of condition due to reduced mobility, nutrition, condition and several forms of homeostenosis, which reduce the capacity of the healing system.
It can also occur due to age, medication and non-directly related comorbidities.
Level 1 factors can have a dramatic impact on the events.
Level 2 wounds are wounds of an event where the lesion itself causes reduction of healing speed. In general, this is the level where the “TIME” acronym is relevant. Usually the damaging events are edema, infection and moisture problems.
Level 3 wounds are wounds which are the result of a repetitive or continuous event, often due
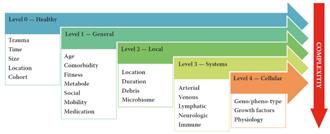
to underlying disease. These diseases are often related to problems with one or more organ systems. The most commonly occurring events are related to the cardiovascular system causing perfusion problems, followed by metabolic issues as diabetes and neurological events. Resolving the cause of the events will make way for the body to heal. In general terms, solving the disease will heal the wound. Often this will be the task of one or more medical specialists.
Level 4 wounds are the result of regulatory problems caused by (epi) genetic events (neoformation) and in some cases antagonistic pleiotropic events like senescence and inflammaging. With the current knowledge, these problems are hard to pinpoint and can usually only be mitigated.
Some general remarks on the levels are that issues on any level can cause issues on all levels, one of the reasons wounds cascade to a more complicated stage over time. It also allows for a ‘heat map’ like analysis due to the correlations between events.
The levels are usually stacked, which means that a level 3 wound also has level 0 - 2 issues.
Data also suggests that there is a Pareto type distribution of the levels with the largest numbers in level 0.
Finally, in the Netherlands we tend to assume
that level 0 requires no treatment, level 1 requires treatment at the level of the GP, level 2 at the level of a specialized nurse, level 3 by a specialist (team) and level 4 is as such not recognised and is usually treated by an appropriate specialist.

Even though the speed of healing is influenced by all levels, not all interventions are equally effective. The best intervention is to remove the cause of a level 3 wound. The rate of success in removal of the cause in level 3 often dictates the wound trajectory. Second best would be to remove an eventual level 2 infection and third is to handle the level 1 issues, like compliance.

The speed at which a wound will heal depends on factors at all levels, but these are usually not recorded in a manner that allows proper analysis. The most obvious example are the Cochrane reviews, which are often inconclusive due to the lack of availability of good research, often due to poor recording of underlying disease. This means that, apart from resolving the cause, finding a single parameter which correlates to the wound healing trajectory is almost impossible. We may have to look at groups of parameters, in something of a network motive fashion. Another suggestion could be to see if we can categorize wound healing speeds in terms of relative size; this may help us in finding outliers.
“Even though the speed of healing is influenced by all levels, not all interventions are equally effective.”

Decentralized clinical trials are designed to optimise patient enrolment and participation by reducing or eliminating the need to travel to specific study sites. This can be achieved using wearable digital health devices.
Traditional clinical trials place a significant responsibility on patients and caregivers. There are also additional costs of transportation, accommodation, and food. Currently patient recruitment onto trials is one of the main challenges in clinical research.
When the pandemic hit in early 2020, the world’s first response was panic. But quickly we learnt how to cope by using the tools already at our disposal.
For the clinical trial industry this included decentralized and hybrid trials. The first decentralized clinical trial was successfully completed over a decade ago, but it took a pandemic for us to fully realise its benefits. As companies raced towards a COVID-19 vaccine, we saw that those who were quick to adopt decentralized approaches reaped the rewards. And we saw how digital solutions opened up access and removed long established barriers to participation.
Now we’ve uncovered the potential of digitalfirst research, it looks as though it’s here to stay. Analysis suggests 2022 will see around 1,300 trials with a decentralised or virtual component1. That’s an increase of almost 30% on 2021 figures, which is very encouraging news and suggests a very positive outlook for the year ahead.
But adopting digital approaches hasn’t been easy and we’ve learnt a lot along the way. To drive more efficient decentralized and hybrid trials beyond the pandemic, there are a few things worth keeping in mind.
Once the realities of the pandemic became clear, patients began to limit their activity and avoid even important trips to trial sites and hospitals. To minimise trial delays or closures, companies turned to decentralized and hybrid approaches. In many cases this meant plugging technology into plans that weren’t originally built for digital. We know that the efficiencies are there when we embed digital tools into the clinical trials process but to see the full benefits of these approaches we need to account for them from the start.
So you’ve included an app in your trial design to collect some patient-generated data. But just because you have this, it doesn’t mean that everyone will want to use it. You need to meet people where they are. While some participants may be happy to do everything through an app, others might prefer to travel to fill out a survey with someone in person. If you’re going to digitise you still need to give participants multiple options so they can pick what works best for them.
Clinicians, patients and sites all have established routines that they’re used to and are comfortable with. Digital needs to be an ally, fitting in with existing processes and workflows rather than disrupting them. By making sure that you’re including every stakeholder in the mapping and implementation of a digital solution, we can bring efficiencies and empower people,
“Clinicians, patients and sites all have established routines that they’re used to and are comfortable with. Digital needs to be an ally, fitting in with existing processes and workflows rather than disrupting them.”
rather than create complications.
Sites and investigators are vital stakeholders who must be a part of the planning, implementation and successful delivery of digital solutions. Unfortunately, the use of the term ‘decentralization’ often neglects the voice and role of sites. Sites should be viewed as trusted partners. We need to engage and empower them so they can usher the use of digital solutions and effectively assist patients on the front lines.
At the end of the day, trials are about patients and their journey. Patients are lending themselves to participate, so in return we should strive to see the process through their eyes and put them at the centre of what we do. If not, we’re doing them a disservice. We must challenge ourselves to learn from our experiences during the pandemic and continue to deliver innovations for them.
1. Kezia Parkins and Andrew Hillman 2022 2022 forecast: decentralised trials to reach new heights with 28% jump Clinical Trials Arena [Online] Available at: https://www.clinicaltrialsarena.com/analysis/2022-forecast-decentralisedtrials-to-reach-new-heights-with-28-jump/
grow at an
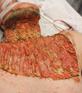
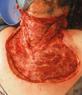
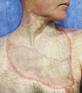
Venous leg ulcers (VLUs) are the most severe stage of chronic venous insufficiency (CVI), defined as the CEAP class C6 (open ulcers). Patients with healed ulcers belong to CEAP class C5.
According to the Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum, a VLU is “a fullthickness defect of skin, most frequently in the ankle region, that fails to heal spontaneously and is sustained by chronic venous disease, based on venous duplex ultrasound testing1.”¹


VLUs represent 70% of all lower extremity ulcerations2 with a prevalence between 0.06% and 2%1. A study within Olmsted County, Minnesota, USA and the Rochester Epidemiology Project (REP)3³estimated the incidence (newly diagnosed venous ulcers) for the time frame 1991-2010 as 0.85/ 1000 personyear, higher than the 0.18/ 1000 person-year incidence reported in the same population for the period from 1966 to 1990. The incidence is much higher in individuals over 60 years of age: it was 8.9/ 1000 person-year in the retrospective cohort study of Olmsted County4. One third of the venous ulcers in the REP study3 had a post-thrombotic etiology. The rates of postthrombotic ulcers, according to the RIETE Registry5, with 3-year follow-up after acute deep vein thrombosis (DVT), were 2.7% at 1 year, 4.4% after 2 years and 7.1% after 3 years. A retrospective study conducted on 3,920 primary care center electronic records in Barcelona6 found the incidence and prevalence of VLUs
doubling during a 4-year period, from 0.5 and 0.8, respectively in 2010, to 1 and 2.2 cases per 1000 person-year in 2014. More than 84% of the VLUs healed and time to healing was shorter in 2014 than before 2010 (19 weeks vs. 453.9 weeks). Only 22.8% of patients were referred for vascular surgery consultation.
The classic cascade of events leading to venous leg ulcers7 includes venous hypertension, chronic inflammation, edema formation and skin changes, from lipodermatosclerosis to active ulcers. The initial understanding of edema formation pathophysiology was based on foundational research by Dr. Ernest Starling regarding the properties and characteristics of the absorption of fluids from connective tissue spaces8. In his thesis, most of the interstitial fluid resulting from arterial perfusion, re-entered the vasculature via the venule, and only 10-20% of interstitial fluid was left to the domain of the lymphatic vasculature for handling. One hundred years later the Starling concept has undergone significant and clinically important revisions9,10. A new potentiator in the realm of systemic fluid homeostasis was identified and continues to undergo extensive in-vitro and in-vivo research and clinical correlations: the endothelial glycocalyx (GCX)11. The renaissance of venolymphatic research and clinical application began with the recognition of the GCX and the importance of GCX benchtop to bedside research and translation continues to grow at an accelerated pace. The relative simplicity of the endocapillary GCX appearance as ‘fine hairs’12 belies its complexity
on the nano scale of glycoproteins, proteoglycans, glycosaminoglycans and additional interlocking components that create a dynamic interactive architecture responsible for macro- and micro vascular and biochemical signaling. Cytokines’ synthesis is involved through shear stress resulting in mechano-transduction and stimulation of the cellular cytoskeleton, provision of a permeability barrier, inflammation mitigation, and coagulation component coverage to prevent inappropriate pathological micro- and macrothrombi development. Several of the critical architectural components include heparan sulfate, hyaluron, albumin, chondroitin sulfate, dermatan sulfate, syndecans, glypicans, and sialic acids. The GCX, having a net negative charge, varies in thickness from 0.2 - 2.0 microns, dependent upon vessel size. It comprises ~25% of luminal volume along the estimated 50,000 miles of vascular and lymphatic length in the adult human; 80-90% of this length is defined as the microvasculature to the level of 5-20 microns. When the GCX is thinned or shed, loss of the GCX functional characteristics is now recognized to be an integral and critical aspect of venous hypertension and associated complications13,14, arterial disease15,16, states of inflammation17, diabetes18, and a multitude of clinical conditions including sepsis, pulmonary edema, COVID-1918A and cancer19
With respect to venous hypertension and subsequent VLU development, the shedding of the GCX results in leukocyte adhesion to the endothelium with increased cellular oxidative stress and vascular permeability leading to the interstitial edema and overload of the lymphatic capacity, widely recognized as phlebolymphedema20. Chronic interstitial edema and lymphatic failure, with persistence of GCX shedding, causes microvascular rarefaction, chronic subcritical tissue ischemia and cellular apoptosis. Hence, VLU healing is improved with resolution of venous hypertension, restoration of lymphatic function, cessation of GCX shedding, and restoration of
a viable and functional endothelial GCX. This is the basis for venolymphatic interventions: manual lymphatic drainage, complete decongestive physiotherapy, lymphatic dynamic and static compression, venous ablation, and lymphedema pumps.
Other factors, contributing to non-healing of VLUs, are associated with environment and with the role of Single Nucleotide Polymorphisms (SNPs), most frequently identified as methylene-tetrahydrofolate reductase (MTHFR) abnormalities that decrease endothelial Nitric Oxide (eNO) production. Several papers have been published regarding the incidence of MTHFR polymorphisms and its involvement in varicose veins and development of thrombophlebitis21-26. MTHFR is a small component of SNPs overall, and the narrative continues to broaden with the identification of additional multiple SNPs. A ‘Polygenic Risk Score’ will likely become more applicable to complete diagnosis of genetic risks27. The full details are beyond the scope of this paper, though research data continue to demonstrate the significance of genetic and epigenetic contributions to an inadequate response by cellular mechanisms in conditions of environmental extremes, or when the human body is required to function at extremes of cellular ability as for example in chronic diabetes, hypertension (arterial and venous), tobacco use, obesity, etc. The redundancy within the genetic is typically protective, though when SNPs reduce/ alter the genetic redundancy of cellular function, the potential for pathological symptoms become increasingly prevalent and prominent28, causing a loss of resilience and increased states of disease29
eNO is crucial to vascular and lymphatic function, neurotransmission and nonspecific host defense30,31. Reduced bioavailability of eNO has been clearly linked to endothelial dysfunction, an early precursor to atherogenesis32,33. eNO ultimately plays roles in
“With respect to venous hypertension and subsequent VLU development, the shedding of the GCX results in leukocyte adhesion to the endothelium with increased cellular oxidative stress and vascular permeability leading to the interstitial edema and overload of the lymphatic capacity, widely recognized as phlebolymphedema20.”A
the synthesis of nucleic acids and byproduct recycling. The synthesis of eNO results from integrated pathways, with folates serving as important cofactors in the transfer and processing of 1-carbon products. Under conditions of oxidative stress or source product deficiencies (among others 5-MTHF; dietary folate, B12, B6, tetrahydrobiopterin or BH4), endothelial nitric oxide synthetase (eNOS) can become ‘uncoupled’32³which leads not only to decreased eNO production, but also to increased production of superoxides, furthering the state of oxidative stress32-36.
Production of eNO has been linked to the structure and function of the glycocalyx in conjunction with caveolae which are essential for maintenance of vascular homeostasis37. Along the entirety of the endothelial surface, caveolae contain endothelial nitric oxide synthetase (eNOS). The glycocalyx components transform the mechanical signals of luminal shear stress into biochemical signals, activating eNOS to produce eNO and decrease reactive oxygen species (ROS) that contribute to cellular oxidative stress37. Shedding of the GCX results in ‘uncoupling’ of eNOS, decreased eNO production, increased ROS production, all consequences recognized to ultimately contribute to cardiovascular diseases such as atherosclerosis and hypertension16,19. Precision, prescriptive, personalized healthcare delivery will be based upon understanding these connections of the patient environment, and both the physiologic and genetic response. From the macro- to the micro- to the nano, integrative and functional health will be based upon macro- and micro-nutrient intake, bioavailability, the gut microbiome, an intact and functional endothelial GCX, and lymphatic/ glymphatic health. Published data is now clearly supportive of the role for appropriate, individualized dosing of adjunctive micronutrients for targeted therapies38-40.
Management of VLUs and lymphedema requires comprehensive care with performance of compression, appropriate treatment of venous insufficiency, and associated lymphatic dysfunction/ lymphedema therapy with Certified Lymphedema Therapists (CLTs)4451,53. Ulcer healing requires adequate nutrition especially in view of the recent discoveries concerning the pathophysiology of chronic venous insufficiency. The Guidelines of the Society for Vascular Surgery and the American Venous Forum41⁴and the European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs42 recommend nutritional assessment in any patient with VLUs with potential malnutrition. Indeed, elderly patients with VLUs might have inadequate intake of proteins, vitamins (A, E, folate, B12, B6, D,C, carotene, and others) and minerals such as zinc57-59. The healing rate can be improved by nutritional supplement60-63
From the multitude of trials with systemic medications studied for their potential benefit in improving VLU healing rates, three products have demonstrated benefit: pentoxifylline, sulodexide and micronized purified flavonoid fraction (MPFF)42.
MPFF has proven microcirculatory protective effects with pronounced inhibition of leucocytes activation and adhesion68-70. In a model of postischemic leukocyte adhesion/ migration and venular protein leakage, MPFF has shown the same strength of effectiveness as the anti-adhesive monoclonal antibodies71. The interaction of MPFF with leukocytes adhesion/ migration was demonstrated in patients with chronic venous disease72. In patients with skin changes plasma vascular endothelial growth
“Precision, prescriptive, personalized healthcare delivery will be based upon understanding these connections of the patient environment, and both the physiologic and genetic response. From the macro- to the micro- to the nano-, integrative and functional health will be based upon macro- and micro-nutrient intake, bioavailability, the gut microbiome, an intact and functional endothelial GCX, and lymphatic/ glymphatic health.”A View Beyond the Horizon; The Re-Emergence of Micronized Purified Flavonoid Fractions for Venous Leg Ulcer Management
factor (VEGF) levels were higher and decreased after treatment with MPFF73.
Hesperidin, an important component of MPFF, is one of the phenolic inhibitors of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase, a key enzyme in the production of reactive oxygen species in the endothelium74. This results in increased bioavailability of eNO.
In the experimental models of venous disease with altered microvascular permeability induced by histamine, bradykinin and leukotriene B4 (LTB4), MPFF exerted a potent effect against leakage of macromolecules75. The inhibition of oxidant-induced leakage by MPFF was efficient to the same degree76.
Medical adjunctive treatment by MPFF has been recognized by international guidelines and summary reviews41,42,43.
The value of MPFF in VLUs therapy was investigated in several randomized controlled studies. MPFF (1000 mg per day) combined with standard compression increased VLUs healing rate and shortened time to healing77-80. In a meta-analysis that included 5 Randomized Controlled Trials (RCTs) with a total number of 723 patients, the estimated relative risk improvement of healing rate at 6 months was 32%81. A retrospective cost-effectiveness analysis, taking into consideration only the direct medical costs, estimated a 45% reduction of treatment costs associated with adjuvant MPFF therapy82, as compared with conventional venous ulcer care.
Venous leg ulcers are the ultimate consequences of chronic venous insufficiency. New discoveries in ulcer pathophysiology assign a special role to glycocalyx and eNO in venous disease development. The management of VLUs has been transformed. The diagnosis of the underlying venous disorder has been greatly improved with the development of the duplex ultrasound technique. Compression therapy options have been enhanced to improve adherence. The novel endovenous ablation and sclero therapy methods for both superficial veins and bionutrient adjunctive therapy has been used with increasing frequency for
patients with vitamins/ minerals deficiencies and oral systemic treatment with MPFF, pentoxifylline and sulodexide (Europe availability) may significantly improve ulcer healing based upon a legacy of prior data and new emerging insights and publications. Undoubtedly, we can now see beyond the horizon and a renewed era for the improved care of patients with VLU and lymphedema.
1. O’Donnell TF, Jr., Passman MA. Clinical practice guidelines of the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF)--Management of venous leg ulcers. Introduction. J Vasc Surg 2014;60:1S-2S.
2. Tatsioni A, Balk E, O’Donnell T, Lau J. Usual care in the management of chronic wounds: a review of the recent literature. J Am Coll Surg 2007;205:617-24e57.
3. Gloviczki ML, Kalsi H, Gloviczki P, Gibson M, Cha S, Heit JA. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for estimating the prevalence of venous ulcer. J Vasc Surg Venous Lymphat Disord 2014;2:362-7.
4. Takahashi PY, Chandra A, Cha SS, Crane SJ. A predictive model for venous ulceration in older adults: results of a retrospective cohort study. Ostomy Wound Manage 2010;56:60-6.
5. Galanaud JP, Bertoletti L, Amitrano M, et al. Predictors of Post-Thrombotic Ulcer after Acute DVT: The RIETE Registry. Thromb Haemost 2018;118:320-8.
6. Berenguer Perez M, Lopez-Casanova P, Sarabia Lavin R, Gonzalez de la Torre H, VerduSoriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre-A time series study (2010-2014). Int Wound J 2019;16:256-65.
7. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med 2006;355:488-98.
8. Starling EH. On the Absorption of Fluids from the Connective Tissue Spaces. J Physiol 1896;19:312-26.
9. Michel CC. Starling: the formulation of his hypothesis of microvascular fluid exchange and its significance after 100 years. Exp Physiol 1997;82:1-30.
10. Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 2014;124:915-21.
11. Martin-Almedina S, Mortimer P, Ostergaard P. Development and Physiological Functions of the Lymphatic System - Insights from Genetic Studies of Lymphedema. Physiol Rev 2021. doi.org/10.1152/physrev.00006.2020
12. Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 1966;25:1773-83.
13. Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J Clin Med 2020;10.
14. Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann Vasc Surg 2018;46:380-93.
15. Mitra R, O’Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE. Glycocalyx in Atherosclerosis-Relevant Endothelium Function and as a Therapeutic Target. Curr Atheroscler Rep 2017;19:63.
16. Weinbaum S, Cancel LM, Fu BM, Tarbell JM. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc Eng Technol 2021;12:37-71.
17. Delgadillo LF, Lomakina EB, Kuebel J, Waugh RE. Changes in endothelial glycocalyx layer protective ability after inflammatory stimulus. Am J Physiol Cell Physiol 2021;320:C216-C24.
18. Broekhuizen LN, Lemkes BA, Mooij HL, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010;53:2646-55.
18A.Rovas A, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. 2021;Angiogenesis 24, 145–157. https://doi.org/10.1007/s10456-020-09753-7
19. Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med 2016;280:97-113.
20. Farrow W. Phlebolymphedema-a common underdiagnosed and undertreated problem in the wound care clinic. J Am Col Certif Wound Spec 2010;2:14-23.
21. Sverdlova AM, Bubnova NA, Baranovskaya SS, Vasina VI, Avitisjan AO, Schwartz EI. Prevalence of the methylenetetrahydrofolate reductase (MTHFR) C677T mutation in patients with varicose veins of lower limbs. Mol Genet Metab 1998;63:35-6.
22. Sam RC, Burns PJ, Hobbs SD, et al. The prevalence of hyperhomocysteinemia, methylene tetrahydrofolate reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. J Vasc Surg 2003;38:904-8.
23. Wilmanns C, Casey A, Schinzel H, Walter PK. Superficial thrombophlebitis in varicose vein disease: the particular role of methylenetetrahydrofolate reductase. Phlebology 2011;26:135-9.
24. Wilmanns C, Cooper A, Wockner L, et al. Morphology and Progression in Primary Varicose Vein Disorder Due to 677C>T and 1298A>C Variants of MTHFR. EBioMedicine 2015;2:158-64.
25. Ekim M, Ekim H. Incidence of the MTHFR polymorphisms in patients with varicose veins. Hippokratia 2017;21:175-9.
26. Lucchi G, Bilancini S, Tucci S, Lucchi M. Superficial vein thrombosis in non-varicose veins of the lower limbs and thrombophilia. Phlebology 2018;33:278-81.
27. Yashin AI, Wu D, Arbeev KG, Ukraintseva SV. Polygenic effects of common singlenucleotide polymorphisms on life span: when association meets causality. Rejuvenation Res 2012;15:381-94.
28. Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 2008;9:465-76.
29. Ukraintseva S, Arbeev K, Duan M, et al. Decline in biological resilience as key manifestation of aging: Potential mechanisms and role in health and longevity. Mech Ageing Dev 2021;194:111418.
30. Bondonno CP, Croft KD, Hodgson JM. Dietary Nitrate, Nitric Oxide, and Cardiovascular Health. Crit Rev Food Sci Nutr 2016;56:2036-52.
31. Chakraborty S, Davis MJ, Muthuchamy M. Emerging trends in the pathophysiology of lymphatic contractile function. Semin Cell Dev Biol 2015;38:55-66.
32. Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol 2002;22:6-13.
33. Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 2017;75:61-70.
34. Zhao G, He F, Wu C, et al. Betaine in Inflammation: Mechanistic Aspects and Applications. Front Immunol 2018;9:1070.
35. Smith SM, Zwart SR. Spaceflight-related ocular changes: the potential role of genetics, and the potential of B vitamins as a countermeasure. Curr Opin Clin Nutr Metab Care 2018;21:481-8.
36. Zwart SR, Gibson CR, Gregory JF, et al. Astronaut ophthalmic syndrome. FASEB J 2017;31:3746-56.
37. Potje SR, Paula TD, Paulo M, Bendhack LM. The Role of Glycocalyx and Caveolae in Vascular Homeostasis and Diseases. Front Physiol 2020;11:620840.
38. Schmidt MA, Goodwin TJ. Personalized medicine in human space flight: using Omics based analyses to develop individualized countermeasures that enhance astronaut safety and
performance. Metabolomics 2013;9:1134-56.
39. Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020;12.
40. Caro-Ordieres T, Marin-Royo G, Opazo-Rios L, et al. The Coming Age of Flavonoids in the Treatment of Diabetic Complications. J Clin Med 2020;9.
41. O’Donnell TF, Jr., Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery (R) and the American Venous Forum. J Vasc Surg 2014;60:3S-59S.

42.De Maeseneer MG, ESVS Guidelines Committee, et al. European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur J Vasc Endovasc Surg (2022) 63, 184-267.
43.Nicolaides AN. The Benefits of Micronized Purified Flavonoid Fraction (MPFF) Throughout the Progression of Chronic Venous Disease. Adv Ther (2020) 37:S1–S5. https://doi.org/10.1007/s12325-019-01218-8
44. Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg 2012;55:122-8.
45. Kankam HKN, Lim CS, Fiorentino F, Davies AH, Gohel MS. A Summation Analysis of Compliance and Complications of Compression Hosiery for Patients with Chronic Venous Disease or Post-thrombotic Syndrome. Eur J Vasc Endovasc Surg 2018;55:406-16.
46. Cheng Q, Gibb M, Graves N, Finlayson K, Pacella RE. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv Res 2018;18:421.
47. Gohel MS, Barwell JR, Earnshaw JJ, et al. Randomized clinical trial of compression plus surgery versus compression alone in chronic venous ulceration (ESCHAR study)--haemodynamic and anatomical changes. Br J Surg 2005;92:291-7.
48. Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ 2007;335:83.
49. Gohel MS, Heatley F, Liu X, et al. Early versus deferred endovenous ablation of superficial venous reflux in patients with venous ulceration: the EVRA RCT. Health Technol Assess 2019;23:1-96.
50. Siribumrungwong B, Wilasrusmee C, Orrapin S, et al. Interventions for great saphenous vein reflux: network metaanalysis of randomized clinical trials. Br J Surg 2021;108:244-55.
51. Gasior SA, O’Donnell JPM, Aherne TM, et al. Outcomes of Saphenous Vein Intervention in the Management of Superficial Venous Incompetence: A Systematic Review and Network Meta-Analysis. Ann Surg 2021.

52. Ghauri AS, Nyamekye I, Grabs AJ, Farndon JR, Whyman MR, Poskitt KR. Influence of a specialised leg ulcer service and venous surgery on the outcome of venous leg ulcers. Eur J Vasc
Saghdaoui
Heatley
- Post-EVRA
Goldschmidt E, Schafer K, Lurie F. A
of superficial venous
S, Gohel
Phlebology 2021;36:48-53.
Surg 1998;16:238-44.
of
J Vasc Surg Venous Lymphat Disord
58.
Senet P. Impact of protein deficiency on venous ulcer healing. J Vasc Surg 2008;48:688-93.
59. Wipke-Tevis DD, Stotts NA. Nutrition, tissue oxygenation, and healing of venous leg ulcers. J Vasc Nurs 1998;16:48-56.
60. Wissing UE, Ek AC, Wengstrom Y, Skold G, Unosson M. Can individualised nutritional support improve healing in therapy-resistant leg ulcers? J Wound Care 2002;11:15-20.
61. Pompeo M. Misconceptions about protein requirements for wound healing: results of a prospective study. Ostomy Wound Manage 2007;53:30-2, 4, 6-8 passim.
62.Hujoel PP et al. Vitamin C and scar strength: analysis of a historical trial and implications for collagen-related pathologies. Am J Clin Nutr 2022;115:8–17.
63. Kim DH, et al. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575; doi:10.3390/nu12020575.
68. Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003;63:71-100.
69. Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol 2005;3:1-9.
70. Coleridge Smith PD. From skin disorders to venous leg ulcers: pathophysiology and efficacy of Daflon 500 mg in ulcer healing. Angiology 2003;54 Suppl 1:S45-50.
71. Korthuis RJ, Gute DC. Postischemic leukocyte/endothelial cell interactions and microvascular barrier dysfunction in skeletal muscle: cellular mechanisms and effect of Daflon 500 mg. Int J Microcirc Clin Exp 1997;17 Suppl 1:11-7.
72. Shoab SS, Porter JB, Scurr JH, Coleridge-Smith PD. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg 2000;31:456-61.
73. Shoab SS, Scurr JH, Coleridge-Smith PD. Plasma VEGF as a marker of therapy in patients with chronic venous disease treated with oral micronised flavonoid fraction - a pilot study. Eur J Vasc Endovasc Surg 1999;18:334-8.
74. Yousefian M, Shakour N, Hosseinzadeh H, Hayes AW, Hadizadeh F, Karimi G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2019;55:200-13.
75. Bouskela E, Donyo KA, Verbeuren TJ. Effects of Daflon 500 mg on increased microvascular permeability in normal hamsters. Int J Microcirc Clin Exp 1995;15 Suppl 1:22-6.
76. Bouskela E, Svensjo E, Cyrino FZ, Lerond L. Oxidant-induced increase in vascular permeability is inhibited by oral administration of S-5682 (Daflon 500 mg) and alpha-tocopherol. Int J Microcirc Clin Exp 1997;17 Suppl 1:18-20.
77. Guilhou JJ, Dereure O, Marzin L, et al. Efficacy of Daflon 500 mg in venous leg ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology 1997;48:77-85.
78. Guilhou JJ, Fevrier F, Debure C, et al. Benefit of a 2-month treatment with a micronized, purified flavonoidic fraction on venous ulcer healing. A randomized, double-blind, controlled versus placebo trial. Int J Microcirc

Debridement is the process of removing any devitalised tissue and bioburden from wounds. This includes necrotic material, eschar, infected tissue, slough, pus, haematoma, and debris. Caution should be exercised when debriding the wound in the community. Many patients are taking anticoagulants, and even the minimal amount of debridement can cause significant bleeding and blood loss1
We have been taught that, in general, you should not undertake a procedure independently unless you are equipped to deal with the worst possible complication, forming the foundation for our clinical practice. However, it is vital to ensure that wound care practitioners are knowledgeable in the most recent advancements in debridement tools and techniques so patient care and clinical outcomes are improved2.
It is important that the method of debridement selected is the most effective for the patient and not limited by the skills of the practitioner. If the practitioner lacks the required skills they should seek support from within their own team, or consider further training if the situation is likely to occur frequently.
Debridement is dependent on the clinical status of the wound, the general health of the patient and the skill and qualification of the healthcare personnel.
Pick a suitable clinical area that has good lighting, access to all dressings and a sterile field to operate in. Ensure the patient is comfortable and provide an explanation to the patient.
Assessment includes a full history of the patient, including duration of wound, exploring comorbidities and any other contributing factors that may highlight the potential aetiology to you.
•
It is important to maintain meticulous documentation at each wound care visit to assess progress and standardised medical photography should also be utilised at each visit.
The main features to document are:
• Location of the wound
Size: considering all dimensions; length, width and depth

Stage: what stage of wound healing is the wound currently in
• Vascular status of the wound
Exudate level
Wound bed
• Periwound
Ascertain the tissue type in the wound1
Viable tissue: this appears with a light pink to red hue and may be moist
•
Epithelial tissue: this tissue is pale pink and may appear white, this normally signifies a healing wound
Granulation tissue: often appears red and dotted in appearance. It is vital to identify overgranulation, as this requires early treatment
Infected tissue: may be inflamed, red, swollen and have a border of erythema or cellulitis surrounding the periwound
• Necrotic tissue: is a result of cell death and may occur when there is concomitant bacteria, viruses, fungi or parasites present. It can also occur if there is a hypoxic wound environment present. Standard treatment generally involves surgical debridement, and antibiotics as per local protocol
Eschar or slough: may be yellow, gray, black or brown in appearance. It may be soft or hard with a leathery appearance. Generally, dry, stable eschar should stay in place
Exposed tendon, ligament or bone: this may appear yellow or off-white and is shiny unless dehydrated. Bone is hard and white unless it is
in a necrotic phase. This often requires surgical reconstruction but in some instances may be temporised with dermal matrices and Negative Pressure Wound Therapy (NPWT).
Most debridement should only be carried out by a trained professional as it carries a considerable risk with it. Debridement, carried out safely, minimizes tissue loss to avoid deep tissue exposure such as bone, joint, cartilage and tendons1. Some gentle debridement devices are licensed and recommended for use of all health care professionals regardless of experience level.
Recent evidence suggests that wound healing is stalled by chronic infection, which consists of the presence of bacteria in a biofilm state. Guidelines recommend adequate wound bed preparation to physically remove the biofilm, with topical products assisting in this process.
Bacterial biofilm is a significant barrier to healing wounds2
• Sharp debridement is the most common form of wound debridement. It is considered a quick and easy method for removing non viable tissue
• Larval Debridement Therapy uses Lucilia sericata (greenbottle fly) larvae to remove necrotic, sloughy and/ or infected tissue, suitable for use in a wide variety of wound types. It should be considered for wounds where rapid debridement is required3
• Autolytic debridement is performed by the application of a prescribed topical agent that chemically liquefies necrotic tissues with enzymes. These enzymes dissolve and engulf devitalized tissue within the wound matrix4
• Enzymatic debridement, also known as chemical debridement, this method is similar to autolytic debridement, but uses proteolytic enzymes instead of the body’s enzymes. These chemical agents break down necrotic tissue. Commonly the enzyme is combined with a dressing that is changed regularly, which softens the tissue and allows for the necrotic tissue to be removed when the dressing is removed. This works faster than autolytic debridement, with little risk to healthy tissue when properly applied
• Jet lavage. Irrigation debridement uses fluid to remove wound debris topical agents, and surface
bacteria. This is still a common debridement method, often followed by another method of debridement. Irrigation is a non-selective debridement technique that is quick and costeffective
Ultrasonic debridement therapy can also remove non viable tissue painlessly, safely and effectively, as well as selectively5
Mechanical debridement is the process of physically removing devitalised tissue from the wound bed. Innovative, evidence based products have been developed to assist with mechanical debridement. Contact debridement pads can be used for mechanical debridement and there are specialised, single-use, monofilament fibre debridement pads and debridement cloths available. The Debrisoft® (L&R) monofilament fibre debridement pad is recommended by the National Institute for Health and Care Excellence (NICE) for use in the community, based on the evidence of effectiveness and estimated cost savings6
• Debridement pads can offer a quick and effective method of debridement that requires no specialist training and can be used in acute and chronic wounds in adults and children7
The management of hard-to-heal wounds by a multidisciplinary team is the best way of improving patient outcomes. The patient’s wishes and comorbidities should also be considered when choosing the type of debridement. Some wounds have the added challenge of requiring many months to heal and may require multiple debridements and/ or several different debridement methods6 However, when debridement is performed correctly and patients adhere to additional treatment recommendations, this procedure can lead to enhanced wound healing, even in chronic or complex wounds8.
techniques in the UK. Wounds UK 2011; (7)1:77-84
6. Vallejo A, Wallis M, McMillan D, Horton E. Use of low-frequency contact ultrasonic debridement with and without polyhexamethylene biguanide in hard-to-heal leg ulcers: an RCT protocol. J Wound Care. 2021 May 2;30(5):372-379. doi: 10.12968/jowc.2021.30.5.372. PMID: 33979219.
7. National Institute for Health and Care Excellence. The Debrisoft monofilament debridement pad for use in acute or chronic wounds. NICE Medical technologies guidance 17. Last updated March 2019 [Internet]. 2014. www.nice.org.uk/guidance/mtg17 [accessed 05/03/2021].
8. Morris, C. Presented at EWMA Conference, Krakow, Poland, 9-11 May 2018. Available from https://lohmann-rauscher.co.uk/downloads/clinical-evidence/EWMA-2018-Debrisoft-v-UCSMolecuLight.pdf. [Accessed 20/06/2022].
9. Manna B, Nahirniak P, Morrison CA. Wound Debridement. 2022 Apr 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 29939659.
10. Swezey L. Types of wound debridement. 2018. WoundEducators.com. Accessed 19th June 2022
11. Fonder MA, Lazarus GS, Cowan DA, et al. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 2008;58:185–206.
This Masterclass Guide is a concise overview aimed at exploring the technique of a mechanical debridement device and how to incorporate this into your clinical practice.
Debridement is the technique by which non-viable devitalized tissue is removed from the wound bed and periwound.
The first step of the wound healing process begins with adequate preparation of the wound bed, and this is a vital step.
Depending on the clinical setting, wound types, and patient factors, different methods of debridement will be required; these include mechanical, chemical, autolytic, and biological.
What Is Debrisoft
?
■ A sterile, single-use debridement device for adults and paediatric wounds, the Debrisoft® monofilament pad and ‘lolly’ utlize patented technology (figure 1), composed of monofilament polyester fibres cut with angled tips
■ Designed for acute or chronic wound care in the community and hospital, the Debrisoft® family group of products has added versatility
■ Debris, slough, bacteria and biofilm are lifted, bound and removed by millions of soft, polyester, monofilament fibres

■ Debrisoft® effectively removes barriers to healing
■ This is an innovative, evidence based alternative to inappropriate debridement methods such as the wet-to-dry technique, which have the potential of damaging healthy tissue and causing discomfort to the patient
■ Available as a pad (figure 3) in two sizes: Debrisoft® pad 10x10cm, for debriding shallow wounds and accessible areas of skin, and Debrisoft® pad 13x20cm, for larger wounds or areas of hyperkeratosis
■ Available as a ‘Lolly’ (figure 4), specially developed to debride hard to reach areas of wound and skin, including deep surgical wounds and for cavity wounds
■ Patented monofilament fibre technology™ aids mechanical debridement (figure 1)
■ The best evidenced tool for mechanical debridement, and the only method recommended by The National Institute for Health and Care Excellence (NICE)1
■ The Medical Technologies Advisory Committee concluded that the Debrisoft® monofilament debridement pad was likely to completely debride appropriate wounds more quickly than gauze or hydrogel and may give earlier visibility of the wound bed1
■ Debrisoft® was considered convenient, easy to use, well tolerated by patients and had a significant reduction in pain immediately after treatment1
a patient with the appropriate wound type. In wounds with hard necrotic tissue, softening by means of autolysis may be necessary before using Debrisoft
can be easily and safely used by any healthcare practitioner in a healthcare environment, with
training is not required for the use of this
can also comfortably self-administer
all emollients are removed. Using only water to moisten the pad, wipe the surface of the wound with the soft fleecy layer
wipe over the wound in a circular motion for 2 to 4 minutes, or use soft, sweeping strokes on the surrounding skin
tissue will bind with the fibres
for each




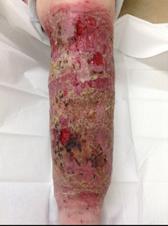



What Is the Evidence?
Debrisoft® a monofilament fibre debridement pad has been found to be a rapid and effective mechanical method of debriding acute and chronic wounds4. This mechanical debridement tool is recommended by NICE for use in the community setting based on the most evidence for effectiveness and predicted cost savings. It is the most well evidenced method of mechanical debridement1 .
■ The success of the Debrisoft® product family in terms of patient comfort and compliance in treatment has been unprecedented
■ Non-invasive Debrisoft® tools are a less painful and safer option compared to potentially destructive debridement techniques in treating wounds in patients with epidermolysis bullosa and pyoderma gangrenosum5
■ Denyer also notes that Debrisoft® could aid in the early diagnosis of certain skin cancers, as these can go undetected when hidden beneath devitalised tissue and debris in the wound area6
■ Can be used by any health care professional
■ Ease of use when self-administered by a patient
■ Avoids travelling to a secondary or tertiary healthcare facility or hospitalization saving time and money
■ Significant savings were shown when Debrisoft® was used in a community and a home setting medical technologies guidance report on Debrisoft®1
■ Before the introduction of Debrisoft® in an audited dataset, the average cost per patient was £64. The cost per patient after Debrisoft® was introduced reduced significantly, to just £204
■ Total cost of wound care products prescribing fell by 14%, in the large audited patient dataset of 4644
■ The use of monofilament fibre debridement pad could reduce wound care products prescribing costs and the subsequent use of costly antimicrobial and negative pressure therapies4
■ “In all patients, almost pain-free and almost complete removal of the fibrin slough was possible by a single application of the debrider without further analgesic procedures... debridement using the debrider represents a non-invasive and therefore safe, almost pain-free alternative, particularly in patients with very painful chronic wounds... This new therapy option can be performed in an out-patient setting without major expenditure in terms of time or materials.” Weindorf & Dissemond, 2014
■ “Most wound debridement requires the skills of specialist practitioners which can be both time consuming and expensive... the new system was found to be a fast and effective method of debridement causing minimal pain to the patients. This new approach to wound debridement could potentially have far reaching benefits to the patient, the nurse and the organisation.” Johnson et at., 2012
■ “When used by a nurse in a community clinic, there were cost savings per patient of £99 for the Debrisoft pad compared with hydrogel, £152 compared with gauze and £484 compared with bagged larvae. When used by a nurse in the home, there were cost savings per patient of £222 for the Debrisoft pad compared with hydrogel, £347 compared with gauze and £469 compared with bagged larvae.” NICE Guidance, 2014
■ Overall cost saving of 20% (included antimicrobial dressings, negative pressure dressings and nonmedicated dressings) using this mechanical debridement device illustrated a 20% cost saving4
■ Compared to other methods of debridement, Debrisoft® may reduce patient consultations with an overall cost savings7
■ Avoiding the inherent costs associated with delayed wound healing is another aspect of cost saving in the use of this product family1




NICE concluded that Debrisoft® is more clinically and cost effective than other debridement methods
Intermittent Vacuum Therapy (IVT) has its origins in space and aeronautical medicine, taking flight from a collaboration between NASA and the Institute of Space Medicine of the German Aerospace Center (DLR). This article explores the potential clinical applications of this innovative treatment.
Peripheral Arterial Disease (PAD) is well known to affect between 15% and 20% worldwide and in Europe we have seen an estimate around 17.8% as described in the PANDORA prevalence study in 2011. In summary, PANDORA was a non-interventional European cross sectional study performed across Italy, Belgium, France, the Netherlands, Greece, and Switzerland, that examined the prevalence of PAD in 9816 subjects with moderate cardiovascular risk and the absence of diabetes or overt vascular disease.
We also know that incidence of chronic wounds has been increasing and is a silent epidemic impacting the quality of life of millions of people worldwide. According to Guest et al., 2015, in the UK alone there are an estimated 2.2 million people currently living with nonhealing wounds.

Chronic wounds, or non-healing wounds, are defined as wounds that are slow to progress through the phases of healing, or display interrupted or delayed healing, due to intrinsic and extrinsic factors impacting on the wound and the individual (Sibbald et al., 2015, Woo et al., 2018). The impact of PAD on chronic wounds and their healing capabilities cannot be merely ignored. Taking into account all the other comorbidities and factors which influence healing, it paints a grim picture, especially for our older generation, when it comes to wound healing. Now the long-term effects postCOVID-19 have also joined the fray, leading to
more complex wound healing. Our biggest challenge with COVID-19 positive patients is systemic coagulopathy, which through hypercoagulation and microvascular occlusion can lead to ischemic stroke, myocardial infarction, venous thromboembolism, acute limb ischemia and pulmonary embolisms (Black et al 2020). This is perhaps where we need to consider adjunctive therapy focused on vascular flow regeneration and lymphatic drainage, to restore the balance of healing.
Why then should we consider vacuumed space technology, also known as Intermittent Vacuum Therapy (IVT), as an adjunctive therapy? According to Weyergans, IVT has its technological origin in space medicine, through collaboration between NASA and the Institute of Space Medicine of the German Aerospace Center (DLR). In the absence of gravity, astronauts in space must undergo negative pressure application every ten hours, to avoid orthostatic complications due to an insufficient baroreflex; in a weightless environment this is the only way to ensure blood flow in the periphery. The development of a lower body negative pressure device (LBNPD) led to the development of the current device, known as the VACUMED® Flow Regeneration System.
The physiological principle behind the VACUMED® Flow Regeneration System is the positive influence of changing cycles of low and normal pressure on the vascular and lymphatic system of the body. The effects of
the low pressure (negative pressure), results in capillary dilatation (enhancing vascular permeability), increased capillarization (formation of small new vessel branches and collateral flow), and therefore improvement in blood flow to the periphery and muscles. The effects of the normal pressure phase results in the increase of venous reflux, increased lymphatic flow, and increased lymphatic drainage. The effects of alternating low pressure with normal pressure causes rhythmic vascular dilation and vascular compression. The device literally functions as a second external heart in promoting arterial flow and venous and lymphatic reflux.
This also explains why IVT with the VACUMED®/ VACUSPORT® device is so effective in sports medicine, and most data currently available is focused on the effect of IVT in sport rehabilitation and management. The ability of IVT to create lymphatic drainage, promote blood circulation and venous outflow, results in oedema reduction as well as the rapid reduction of haematoma. This results in acceleration of wound healing, improved tissue management and shortening of rehabilitation time in athletes.
IVT has been effective in the management of fatigue-related pain, half muscle stiffness (Type 1 A); muscle fibre tear (Type 2A, 3A, 3B), (Sub-) total muscle tear; avulsion, and sinewy avulsion (Type 4), by shortening individual return to play by 30 to 50% while maintaining capillarization and shortening recuperation time. The device is used to shorten the rehabilitation period postoperatively or post-traumatically, as well as in cases of special indications. This also applies to all types of muscular injuries, and to the rapid recovery of full performance after training or competitions.
Everything in life is about blood flow and lymphatic drainage. Without the ability to have oxygen carrying arterial flow, active venous
return, and lymph drainage, the ability of the body to successfully heal itself is severely compromised. In this regard, IVT might just be that lifesaving technology we have been looking for. IVT can decrease chronic oedema associated with secondary oedema in the long term, leading to a decrease in both venous oedema and lymphoedema, as well as secondary lymphoedema in the postoperative acute phase. This is achieved through the mechanical passive lymph drainage of the device, and can be used in addition or as an alternative to manual lymph drainage. The implication herewith has a major impact on wound healing post operatively, but also in decreasing oedema prior to surgery.
Perhaps the biggest impact possible is the implementation of IVT within the wound care environment. Within the diabetic foot our aim is to save limbs and prevent amputation. In aworld where not every patient has the means or ability to timeously see a vascular surgeon, IVT might just be that limb saving, lifesaving therapy that can change the future of vascular interventions. It comes as no surprise that IVT is indicated for PAD (pAVD I-IV according to the Fontaine classification) as well as Diabetic Foot Syndrome (DFS). This is achieved through revascularisation, by stimulation of the endothelium (capillarisation and Nitrous Oxide (NO) release), blood flow promotion (flow increase), and tissue oxygenation (tissue management). If we can improve blood flow and revascularisation, promote venous outflow by active vascular dilatation and compression, and improve the physiological muscle pump by increasing collagen synthesis, we can decrease oedema, improve walking distance and restore the oxygen balance within the body; all of which has a significant impact on wound healing. This also means that IVT can have a significant impact on Chronic Venous Insufficiency (CVI) CEAP I-6, through the same mechanism of promoting blood circulation and venous outflow through active vascular dilatation and compression.
“If we can safe one more limb, improve a patient’s quality of life by improving blood flow, promoting revascularization, decreasing oedema and lymphoedema; why should we not consider space technology?”Vacuumed Space Technology: A Farfetched Dream or a Life Changing Technology?
(MDR) authority has recently approved more than 40 benefits/ indications for IVT with the VACUMED®/ VACUSPORT® device, and even though there have been some early studies such as Hageman et al., (2019), showing no significant evidence through a small randomized controlled trial (RCT), the overwhelming evidence ranges from a Class 1B to 4 is indicative of the opposite.
If we can save one more limb, improve a patient’s quality of life by improving blood flow, promoting revascularization, decreasing oedema and lymphoedema; why should we not consider space technology? Perhaps it is time that we look to the future and focus on prevention, by improving a patient’s vascular status to prevent associated diseases.
Future studies on the impact of IVT on COVID-19 patients should be considered, and perhaps this would be the exploration of the great unknown. IVT space technology a farfetched dream or lifesaving technology? !e real question is what do we have to lose for us to decide? Perhaps its time that we sit up and take notice of what can be done without traumatic surgical interventions or as an adjunctive in improving our post-surgical outcomes.
Vacuumed Space Technology: A Farfetched Dream or a Life Changing Technology?The burden of hard-to-heal wounds continues to grow and has a disproportionate impact on resources1. Chronic wounds account for two-thirds of the total cost of wound care within the U.K. (£6.5 bn) and costs continue to increase1. Despite following best practice, many wounds do not heal. New innovative technologies should be considered in everyday clinical practice, to expedite healing and improve the quality of life for the patient. Often it is wound pain which the affects patients’ quality of life most significantly and its management should avoid the use of analgesics where possible.
This article will explore how electrical stimulation therapy (EST) has a unique combined effect of reducing wound pain and stimulating wound healing and is in fact one of the most evidence based modalities in wound management today. A new device, Accel-Heal Solo, is now available that supports much easier adoption into frontline clinical practice. Accel-Heal Solo provides the benefits of EST, delivered by a single-use, pre-programmed, wearable, easy to use device. Observational studies on the predecessor device, Accel-Heal, report significant reductions in wound pain within 2 weeks of treatment and accelerated healing rates, which translate into significant cost savings and improved patient quality of life. Improved outcomes are also shown in real life settings and individual case studies will be shared.
Despite the huge number of dressings currently available, there has been surprisingly little real innovation in wound management over the last 20 years2 and the financial burden of hard-to-heal wounds on the UK’s National Health Service (NHS) continues to grow1. Recent prevalence of wounds was estimated to be 3.8 million among the adult population of the UK, increasing year on year2. Guest et al (2020)¹determined that 51% of chronic wounds remained unhealed in the study year, accounting for two-thirds of the total cost of wound management, which is estimated to be £8.3bn per annum. The majority of patients are managed in the community setting, with health care professionals (HCPs) accounting for 53% of the costs. With prevalence and costs increasing, this will continue to have a significant effect on an already depleted NHS workforce and resources. Some expert panels3
have even suggested that globally ‘wound care is in crisis’.
The impact for patients and their family of living with a chronic non-healing wound is also well documented4-9, many of whom live without any hope of improvement, whilst suffering pain, non-healing, infection, sepsis, risk of amputation and death10-11.
Challenges in management centre upon achieving healing and the avoidance of adverse events such as infection, sepsis and amputation, together with symptom management, including pain, exudate and irritation, to improve quality of life. Best practice should be instigated to include: holistic management of the wider factors that delay healing; prevention of wounds; revascularisation; lifestyle changes such as smoking cessation and nutrition; reducing and

managing infection; wound bed preparation/ management; pressure off-loading/ reduction and pain management10,12-19.
However, despite following best practice, many wounds still fail to heal. Alternative approaches need to be considered, rather than by simply applying yet another topical dressing, many of which have been around for 50 or 60 years2, and in the main, focus on exudate and antimicrobial management, to facilitate the body to self-heal, rather than being an interactive treatment to stimulate cell processes and promote wound healing. Most wounds fail to heal due to underlying conditions of the patient and within the wound itself, and the primary dressing alone is unlikely to address this.
The National Wound Care Strategy Programme (NWCSP) has been commissioned by NHS England and NHS Improvement for pressure ulcers, lower limb and surgical wounds, and seeks to improve care for people with wounds, to reduce patient suffering, improve healing rates and prevent wound occurrence and recurrence.
Leg ulcer assessment, diagnosis and treatment are included in the 2022/ 2023 Commissioning for Quality and Innovation (CQUIN)20, in the community setting, in order to increase the number of patients receiving high compression therapy if indicated. However, despite compression therapy being one of the most evidence based therapies for venous leg ulcers, it is often not tolerated by patients due to the pain21, with evidence suggesting that 40% of HCPs would remove or reduce compression if the patient had significant pain22.
How can we do things differently to reduce patient suffering, promote healing, improve clinical outcomes and meet the new CQUIN targets?
Innovation is being supported for use across the healthcare system in the NHS Long Term Plan23, and will be central to securing transformation and improved patient outcomes. Fast adoption of cost-effective new technologies will be accelerated23, so that proven innovations get to patients faster.
So, the green light has been given to HCPs, to use alternative approaches in managing hardto-heal wounds, rather than just using another dressing.
This article will explore EST and the compelling evidence base behind it. EST has been used for many years for wound management, but widespread adoption has previously been challenging due to the format it was available in.
Whilst very efficacious, devices were complicated clinic based units, requiring specialist training and could not be used in conjunction with preferred dressing regimes and compression therapy if indicated.
The innovative treatment (Accel-Heal Solo) has now made frontline adoption of EST possible. Accel-Heal Solo is a single-use wearable device that delivers a fixed 12-day programme of subsensory electrical stimulation therapy to hard-to-heal wounds to relieve pain and accelerate healing. The article will further discuss the mode of action, benefits, application, indications and contra-indications for using Accel-Heal Solo together with the growing evidence that is available.
Human physiology is electrochemical in nature. Within the skin, a stream of Na+, K+ and Clions moving in opposite directions create a difference in voltage between the surface of the epidermis and the deeper layers, establishing a stored electrical potential or ‘skin battery”.
“Accel-Heal Solo is a single-use wearable device that delivers a fixed 12-day programme of subsensory electrical stimulation therapy to hard-to-heal wounds to relieve pain and accelerate healing.”
After wounding, microcurrents flow into the wound site, establishing a ‘current of injury’24, which stimulates cellular activity such as migration and activation of gene expression in macrophages, endothelial cells, fibroblasts and keratinocytes25, as part of normal wound healing (see Figure 1). The current of injury extends up to a radius of 2-3mm around the wound26. As the wound closes, the current of injury progressively reduces.
Due to adverse events occurring within the wound such as the presence of foreign bodies; accumulation of slough and necrotic tissue or following the development of biofilms and/ or infection, many wounds become stuck in the inflammatory phase. In chronic non-healing wounds, microcurrents are thought to dissipate and become dysfunctional or completely absent27.
Application of electrical stimulation therapy is effectively restoring a normal process in wound healing to mimic the current of injury, which has become “exhausted” due to the chronicity of the wound (see Figure 2). So, far from being a sideshow or a curiosity, electric fields are established as part of the fundamental mechanisms of cell and tissue growth and control28.
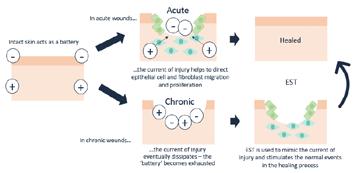
EST has been used in medicine for many years, for example; in cochlear implants, cardiac pacemakers, spinal cord nerve stimulation and facial therapies for aging skin. The level of electricity delivered varies, depending on the type of stimulation required.
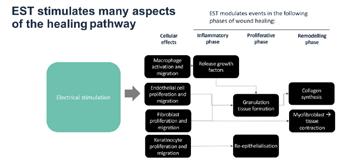
Where strength-duration of the stimulus exceeds the threshold for nerve activation, electrical stimulation causes either a sensory effect (that can range from tingling to ‘pins and needles’), or a motor effect such as a muscle twitch, some of which can be painful for the patient25. Electrical stimulation devices for wound healing can be effective at a much lower level of stimulus (see Figure 3).
Figure 3; EST: Where strength-duration of the stimulus exceeds the threshold for nerve activation electrical stimulation causes either a sensory effect (that can range from tingling to ‘pins and needles’) or a motor effect such as muscle twitch.
The electrical impulses of Accel-Heal Solo remain in the subsensory domain.
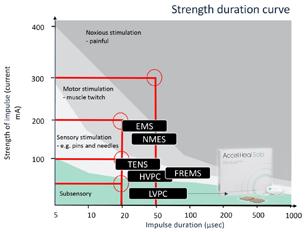
Electrical stimulation therapy (EST) is one of the most widely evidence-based therapy areas in wound management. The extensive evidence base includes nine meta-analyses30-38, eight systematic reviews, and over 35 RCTs, that describe the efficacy of EST in wound management. Although the EST described in these studies is delivered via a variety of formats and stimulation parameters, this evidence base does provide a high level of validity and confidence in the science, mode of action and clinical effect of this technology platform, to reduce pain and stimulate wound healing.
Electrical stimulation has been recognised for use internationally in guidelines and recommendations from the European Wound Management Association and the National Pressure Ulcer Advisory Panel16,39 for a range of chronic wounds, such as pressure ulcers, leg ulcers and diabetic foot ulcers.
Accel-Heal Solo is a class IIa medical device which delivers low voltage biphasic monophasic pulsed current (LVBMPC) electrical stimulation therapy to the wound, mimicking levels seen in the body’s natural systems. The device delivers repeated treatment sessions, each lasting 28 minutes and 40 seconds, with a resting period repeated several times daily throughout the 12day treatment period. Meta-analyses34,43 have shown that EST devices, like Accel-Heal Solo, that deliver a “pulsed current” stimulation are more efficacious than other types of devices.

Accel-Heal Solo is small and discreet, about the size of a car key fob, and can be worn unobtrusively by the patient in all settings, without the need to attend specialist clinics. Accel-Heal Solo is worn continuously over the therapy period and is used alongside standard therapy to ensure wound bed preparation strategies are continued according to best practice40-42, and compression therapy if
practice40-42, and compression therapy if indicated for leg ulcers.
Accel-Heal Solo (see figure 4), is the next generation of Accel-Heal and offers all the benefits of the original device (which needed changing every 48 hours) while providing the therapy using one device which runs for the duration of the 12-day treatment. While the 12-day programme of electrical stimulation is identical, the use of a single device ensures a more regulated and controlled delivery of the therapy and simpler management by clinicians, carers and patients alike. Accel-Heal Solo also provides a new LED light feature, to provide the patient/ carer/ clinician information regarding the operational status, and a clip and strap to secure the device.
Accel-Heal Solo is recommended for:
• Any wounds not healing as expected including leg ulcers, DFUs, post-operative wounds, pressure ulcers and burns.
• Patients with unmanaged pain.
• Patients who are unable to tolerate any or high compression therapy due to pain.
• Patients who are unable to tolerate treatment regimes such as debridement Patients with high risk of delayed healing such as immunocompromised, diabetes, ischaemia; recurrent wounds.
“Electrical stimulation therapy (EST) is one of the most widely evidence-based therapy areas in wound management.”
What Is the Role of Electrical Stimulation Therapy?Indications and contraindications for use are provided in the IFU: LINK Figure 4: Accel-Heal Solo
Accel-Heal Solo is a current controlled device, which monitors current flow through the electrodes and automatically adjusts the voltage to ensure the required current program is delivered, regardless of variables, such as skin texture and distance of electrode pads. This enables the electrode pads to be placed at some distance from the wound edges, and the device will still deliver the prescribed dose of EST44, thus enabling the pads to be placed away from pressure points, for example sacral wounds and diabetic foot ulcers to the foot plantar (see figure 5).
Hard-to-heal wounds may have an initial response to Accel-Heal Solo therapy, but as with any wounds, can then deteriorate or become stalled again. This can be due to untoward events in the wound such as infection, or a general deterioration in the general health of the individual. In the author’s experience, and that of key opinion leaders45, although the majority of cases only require one Accel-Heal Solo therapy to expedite complete healing of the wound, those patients who had a positive response to the initial treatment, which then become stalled or exacerbated, might benefit from a second application of the Accel-Heal Solo therapy. It is recommended to wait approximately 4 weeks after initiating the first therapy, to enable the wound to kick start and
to demonstrate the early clinical signs of improvement that is so frequently observed.
In chronic wounds, there is strong and growing evidence demonstrating that Accel-Heal Solo can reduce pain, kick start the healing process, improve quality of life for patients, and provide economic benefits28,46-69
NICE70, advocates to consider using alternative non-pharmacological approaches to manage chronic pain. Analgesia is known to have many adverse effects, and often patients are reluctant to take them, or they are ineffective.
One widely used device for pain management across a range of co-morbidities, is transcutaneous electrical nerve stimulation (TENS), involving the use of milliamp current to reduce pain and muscle spasms. The stimulus exceeds the threshold to cause a deliberate sensory effect (see Figure 3), so is an intentionally high stimulus, adjusted by the individual, to interfere with/ block the gate control for pain signals in nerve fibres. It can take some time for tolerance to be established, and causes tingling through to throbbing and even discomfort71
“In chronic wounds, there is strong and growing evidence demonstrating that Accel-Heal Solo can reduce pain, kick start the healing process, improve quality of life for patients, and provide economic benefits.”
Is the Role of Electrical Stimulation Therapy?
However, TENS is not a cure for the pain, and often only provides short-term relief while the TENS machine is being used. Alternatively, a microcurrent device, such as Accel-Heal Solo, acting at a much lower level of stimulation, (1000 times weaker than TENS71), restores the natural endogenous current to tissue, in order to provide a healing effect, and in doing so also has an analgesic impact at the localised level by dampening down the inflammatory response71.
Evidence has highlighted that Accel-Heal reduces pain within a few days and sometimes even within a few hours of application, and this pain reduction is sustained following the 12-day therapy.
The exact mechanism of pain reduction using EST is unknown, but it appears that the pain reduction is often noted well before wound healing is achieved, indicating a more active and specific effect25.
A randomised gene expression analysis72, demonstrated that after 48 hours of stimulation, Accel-Heal was found to affect gene expression in human skin. In particular, Accel-Heal down-regulated 25 proinflammatory genes that are implicated in painful, chronic ulcers and in inflammation. This change in gene expression may represent a dampening effect and may be of benefit to wounds. This supports the hypothesis that Accel-Heal can help move a wound from the inflammatory phase into a healing phase. As inflammation is linked to pain, this study may also suggest a mechanism behind the pain reduction that is seen in clinical practice.
The evidence demonstrating the positive clinical outcomes regarding pain reduction using Accel-Heal therapy is significant. Information has been extrapolated from previous studies, to include any patients/ wounds who had their pain score recorded (visual analogue score (VAS) range 0-10), prior to Accel-Heal therapy, at the end of the 12 day
treatment and at the end of the study period28,51,53,55,58,62,66-69
71 patients from 11 studies were included in the analysis, and had been evaluated for usage of Accel-Heal EST for a period of between 4.3 to 21 weeks. Many patients had pain scores >5, and were taking several types of analgesia, and suffering sleeplessness and social isolation.
The data demonstrated a mean pain score of 6.81 (VAS) prior to therapy, reducing to a mean of 2.62 (61.5% reduction) after the 12 day therapy and reducing to a mean of 0.4 (94.2% reduction) by the end of the evaluation (see Figure 6).
This pain reduction had a significant impact on many patient’s lives, with many reducing their analgesia, reporting improvements in their mobility and social lives and sleeping.
A range of evidence has demonstrated the positive impact Accel-Heal has on wound size in hard-to-heal wounds. In three observational studies28,51,58, involving 37 non-healing wounds, healing was achieved in 84-90% within 20 weeks. 27% (n=10) of wounds had been present for over one year prior to Accel-Heal therapy.
To validate the healing potential even further, information has been extracted from several previous studies to include any patients’ wounds who had their wound dimensions recorded prior to Accel-Heal therapy, following
“The evidence demonstrating the positive clinical outcomes regarding pain reduction using Accel-Heal therapy is significant.“
Figure
the treatment and at the end of the study period28,46,51,53,55,58,60,62,65-69. 120 patients/ wounds from 14 studies were analysed. The studies included a range of complex non-healing wounds, including venous leg ulcers (failed to heal despite compression therapy), DFUs, post-operative wounds and arterial ulcers. Some wounds were extensive and had been present for many years. Patients had been seen for the evaluation process for a period of between 12 days to 182 days. The analysis demonstrated a mean wound size of 17.25 cms square (range 0.04 - 133), prior to AccelHeal therapy. Following the therapy (assessed between 12 days to 8 weeks), the mean wound size was reduced to 12.8 (reduction of 25.8%). At the end of the evaluations, the mean size was 4.97 (reduction of 71.2% (see Figure 7).
The combined effect of the pain relief and wound size reduction results are illustrated (see Figure 8) to demonstrate how applying AccelHeal to static wounds results in immediate and significant pain reduction followed by wound healing over a 12 week period. Accel-Heal restores the normal wound healing process, which relieves the pain and stimulates healing.
Several economic studies have demonstrated the cost effectiveness of using Accel-Heal46-49 One study51 demonstrated a decrease of 74% in district nursing time over the 20 week study period (see Figure 9), resulting in
12 days following therapy, and at the end of the study
weeks) (n=71)
Reduction in wound size following Accel-Heal 12-day therapy
Reduc�on in pain following AccelHeal
Reduction in pain following Accel-Heal 12-day therapy
day
Figure
Mean reduction in pain and wound size after Accel-Heal is applied to non-healing wounds at week 0
Mean reduction in pain and wound size after Accel-Heal is applied to non-healing wounds at week 0
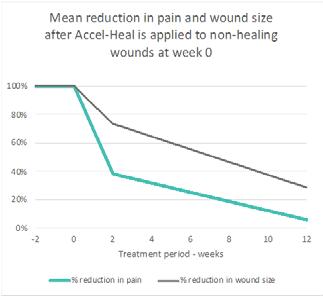
cost-efficiencies in nursing time and cost savings in dressings and bandages. Analysis of a previously reported RCT47 determined that wounds being treated with Accel-Heal, healed on average 3.6 weeks faster vs standard care, resulting in cost savings of £936 per patient on average.
An observational study (n=14) was undertaken in a community trust in Scotland, to demonstrate the effectiveness in pain reduction and wound size reduction for patients with hard-to-heal wounds using the 12-day Accel-Heal therapy. Data was collected prior to the application of
Accel-Heal, following the 12-day therapy, and every 4 weeks, for up to 20 weeks. Wound aetiology included venous leg ulcers (VLUs) (n=13) and unknown aetiology leg ulcers (n=1).
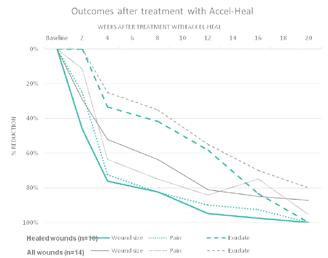
57% (n=8) patient’s wounds had pain, with a mean pain score of 3.14 (range 0-10), and 29% (n=4) had a pain score >5. On completion of the Accel-Heal therapy, the mean pain score was reduced to 2.79 (range 0-10) demonstrating an 11.4% reduction. At the end of the study period, the mean pain score was reduced to 0.14 (range 0-2), demonstrating an overall pain reduction of 95.5% during the study period (see Figure 10). The study also demonstrated a mean wound size reduction of 29% and 87% within 2 weeks and 20 weeks of commencing the therapy respectively. 71.4% (n=10) of all wounds healed within the study period.
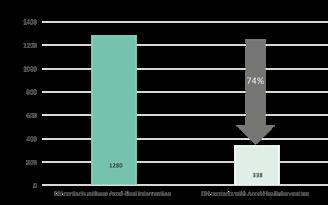
A 66 year old gentleman was in hospital in Kuala Lumpar with a DFU to the lateral aspect of the right foot, which had been present for 2 years. Past medical history included Diabetes Mellitus and renal transplantation.
Despite multiple sessions of wound debridement, the wound showed no improvement. Treatment with Accel-Heal therapy commenced (day 0) on 21/04/21, and the wound measured 105 cm square with approximately 90% slough and exposed tendon (see Figure 11) and moderate purulent exudate. Dressings continued with protective absorbent dressings. His pain score was 4/10 (VAS), which was affecting his sleeping.
On day 9, the wound had reduced in size, measuring 97.5 cm square (see Figure 12).
The pain score reduced to 2/10 (VAS), with a significant improvement with his sleeping.
Fourteen weeks after commencing Accel-Heal therapy, the wound had improved considerably
and measured 40 cm square with 50% slough, demonstrating a 62% reduction in size (see Figure 13), and a pain score of 1/10 (VAS).
A 90 year old female was being seen at home by community nurses in the UK, for a non-healing VLU to her tibial crest following a traumatic injury 4-6 months previously. Past medical history included dementia. Her ankle brachial pressure index (ABPI) was within normal limits, but she had a history of intolerance to compression therapy due to the presence of
“Wounds being treated with Accel-Heal, healed on average 3.6 weeks faster vs standard care, resulting in cost savings of £936 per patient on average.”
What Is the Role of Electrical Stimulation Therapy?Figure 9: Reduction in district nursing visits with/ without Accel-Heal intervention for the 20 week period52 Figure 10: Wound size reduction and pain reduction of observational study undertaken in Scotland using Accel-Heal therapy (n=14).
Figure 11: Day 0 - Extensive
DFU to lateral aspect right foot prior to Accel-Heal therapy.
Figure 12: Day 9 - DFU to lateral aspect right foot following Accel-Heal therapy.
Figure 13: Week 14 - DFU to lateral aspect right foot following Accel-Heal therapy.
high pain levels. Prior to Accel-Heal therapy (day 0), the wound measured 24.75 cm square, with 30% slough and evidence of friable tissue (see Figure 14), requiring twice weekly visits for application of honey dressings and high compression therapy. Her pain score was 5/10 (VAS).
Following the 12-day Accel-Heal therapy (day 14), the wound measured 10.25 cm square (reduction of 58.5%), and dressings were reduced to weekly. The pain score reduced to 1/10 (VAS).
Within 12 weeks of commencing Accel-Heal, the wound healed with no pain (see Figure 15).
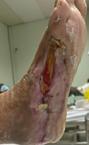
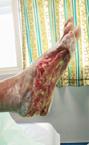
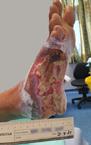
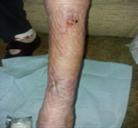
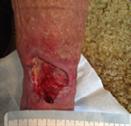
Patient feedback has been significantly positive55,61-63,66-67. Patients who were previously taking opiates have been able to remove their analgesia and reported improved sleeping and mobility. Some testimonials from patients include:
“If I hadn’t had the device put on I think I would have been struggling.”
“I would recommend it to anybody... If I had to have the treatment again I would have it willingly because I couldn’t stand to have that pain again.”
“For a long time we were getting nowhere, I feel like because of that I lost a few years as it was a real struggle, so now I’m delighted to start improving… I have a great family around me who are all so pleased with the progress, just ask my daughter in law, she’s thrilled.”66
EST needs to be considered for use by clinicians, in order to replenish the diminished/absent microcurrent signalling in chronic wounds, which is so vital in the complex healing cascade.
There is a wealth of evidence to demonstrate the significant improved clinical outcomes and economic benefits of using Accel-Heal Solo for hard-to-heal wounds. Benefits include early relief of pain, reduced inflammation, oedema and exudate and stimulation of the healing cascade. These benefits result in improved mood, reduced anxiety and sleeplessness and improved mobility for patients, many of whom have given up any hope of their wound/s healing and suffer endless unmanaged pain.
Accel-Heal Solo also enables tolerance to other gold standard treatments such as debridement, wound cleansing and compression therapy, which are frequently poorly accepted by patients due to the pain and inflammation.
Accel-Heal Solo is a cost effective, easy to use EST, which is readily available for use in the UK, for use in hard-to-heal wounds to relieve pain and accelerate healing. The unique, compact device is ideal for use in a variety of settings, and the ease of use enables it to be accessible for patients, carers and clinicians.
Figure 14: Day 0 prior to Accel-Heal treatment Figure 15: Wound healed within 12 weeks of commencing Accel-Heal For more case studies, see the following link: LINK1. Guest JF, Fuller GW, Vowden P (2020). Cohort study evaluating the burden of wounds to the UK’s national Health Service in 2017/2018: update from 2012/2013
Fletcher J (2021) Has anything changed? Wounds UK. 17(4): 6-9.
Murphy C, Atkin L, Swanson T et al (2020). International consensus document. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: wound hygiene. J Wound Care; 29 (Suppl 3b):S1–28.
Arshad M, Arshad S, Arshad S et al (2020). The Quality of life in Patients with Diabetic Foot Ulcers. Journal of Diabetes and Metabolism 11 (2) 1-2
González-Consuegra RV, Verdú J (2011) Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs67(5): 926–44
Gorecki C, Brown JM, Nelson EA et al (2009) European Quality of Life Pressure Ulcer Project group. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 57(7): 1175–83
Polikandrioti M., Vasilopoulos G., Koutelekos I., et al (2020) Quality of life in Diabetic Foot Ulcer: Associated Factors and the
//www.nice.org.uk/guidance/cg147/resources/peripheral-arterial-disease-diagnosis-and-management-pdf -35109575873989
19. Fletcher J (2022). Why do we prevent pressure ulcers and treat leg ulcers? Wounds UK. 18(1):6-9
20. NHS (2022a). Commissioning for Quality and Innovation (CQUIN): 2022/23. NHS England and NHS Improvement. Available on-line at:-https://www.england.nhs.uk/wp-content/uploads/2022/01/B1477-i-cquin-22-23-march-2022.pdf
21. Dhoonmoon P (2020). A multi-professional approach to improve healing rates of complex patients under the care of an integrated lower limb service in an inner London Borough. Presented at EWMA virtual conference.
22. Atkin L and Martin R (2020). An audience survey of practice relating to pain in the management of chronic venous leg ulcers. Br J Community Nurs. 25(Sup12):S20–S24. https://doi.org/10.12968/bjcn.2020.25.Sup12.S20
23. NHS (2019) The NHS Long Term Plan. Available on-line at https://www.longtermplan.nhs.uk/wp-content/ uploads/2019/08/nhs-long-term-plan-version-1.2.pdf
24. Kloth L (2014). Electrical stimulation technologies for wound healing. Adv Wound Care 3(2): 81–90
25. Milne J., Swift A., Smith J., Martin R.(2021) Electrical Stimulation for Pain Reduction in Chronic Wound Healing.
Journal of Wound Care 30 (7) 2-13
26. Tadej M, Young S, Hampton S (2010). Accel-Heal®: a new therapy for chronic wounds. J Comm Nursing 24(5)16-20
Kloth L, McCulloch J (1996). Promotion of wound healing with electrical stimulation. Adv Wound Care 9: 42–5
Ovens L (2019a). Application of Accel-Heal for patients with chronic venous leg ulcers: an evaluation in a community UK NHS trust. Wounds UK 15 (2) 110-116
Hooker D, Prentice W (2002). Ch 5 In. Therapeutic Modalities in Rehabilitation. 4th Edition. McGraw Hill Medical. www.infinityrehab.com
Avendano-Coy J, Lopez-Munoz P, Serrano-Munoz D et al (2021). Electrical microcurrent stimulation therapy for wound healing: A meta-analysis of randomized clinical trials. J Tissue Viability S0965-206X(21)00132-7.
AroraM, Harvey L, Glinsky J et al (2020). Electrical stimulation for treating pressure ulcers. Database Syst Rev; 1 (1): CD012196. Cochrane review
Chen Z, Chen Z Y, Li G (2020). Electrical stimulation as an Effective Adjunctive Therapy for Diabetic Foot Ulcer: A Meta-analysis of Randomized Controlled Trials. Adv Skin Wounds Care 33 (11) 608-612
33. Girgis B and Duarte J (2018). High Voltage Monophasic Pulsed Current (HVMPC) for stage II-IV pressure ulcer healing. A systematic review and meta-analysis. J Tissue Viability; 27(4):274-284
34. Khouri C, Kotzki S, Roustit M et
with venous leg ulcers (VLUs). Presented at EWMA
59. Alktebi S and AlAdab A (2022) Use of a novel electrical stimulation device to kick start healing in two patients with lower limb ulcers complicated by diabetes mellitus. Presented at WUWHS.
60. Kurz, P, Danner G, Martin R (2022). Clinical evaluation of the response rate to a continuously active, single-use electrical stimulation device in static non-healing wounds. Accepted to EWMA
61. Kiernan C, Strapp H, Cafrey B et al (2020). Effectiveness of a single-use, portable, electrical stimulation device in vascular foot ulcers. Presented at EWMA.
62. Terrill P and Ovens L (2022). An innovative approach to manage pain and stimulate healing in arterial ulcers using electrical stimulation therapy. Poster accepted to EWMA.
63. Turner N and Ovens L (2017b). Clinical outcome results and quality of life improvements using electroceutical treatment - Patient perspectives. Presented at EWMA.
64. Cancela C, Cruz M, Kaha E et al (2022). Ease of use of wearable, single-use electrical stimulation device for the management of hard-to-heal wounds. Accepted to EWMA. 65. Louison P (2015). Management of recurrent venous leg ulcer with electroceutical therapy* to improve pain, expedite healing and reduce risk of recurrence. Presented at EWMA, London. 66. Layflurrie and Ovens (2021) . What can we do differently. Presented at Wounds UK 67. Nair H (2022). Powering the progression of hard-to-heal with electrical stimulation: anobservational analysis of wounds treated with Accel-Heal. Awaiting publication. 68. Ovens L (2022). The way forward: new technologies need to be adopted to improve healing and reduce pain in patients with chronic wounds. A case study to demonstrate the effectiveness in pain reduction and wound size using a novel 12-day, compact electrical stimulation therapy device (Accel-Heal Solo) for management of a recalcitrant arterial foot ulcer, in the community. Awaiting publication, data on file. 69. Ovens L (2014). Electroceutical therapy to manage complex leg ulcers: a case series of three patients. Wounds UK; 10 (2) 78-83 70. NICE Guidelines (NICE). Medicines optimisation in chronic pain. 2019; (accessed April 2022):1-10. https://www. nice.org.uk/advice/ktt21 71. Atkinson M (un-published). Pain ease microcurrent therapy treatment in subjects with period pain (dysmenorrhoea). A pilot study. Available on line at (accessed 22/04/22) :- http://www.painmasterhellas.gr/pdfs/clinical_studies/Clinical%20 Trial%20Period%20Pain.pdf 72. Lallyett C, Yeung C-YC, Nielson RH, et al (2018). Changes in S100 Proteins Identified in Healthy Skin following Electrical Stimulation: Relevance for Wound Healing. Adv Skin Wound Care;31(7):322-327.
“The unique, compact device is ideal for use in a variety of settings, and the ease of use enables it to be accessible for patients, carers and clinicians.”
■ Human physiology is electrochemical in nature. Within the skin, a stream of Na+, K+ and Cl- ions moving in opposite directions create a difference in voltage between the surface of the epidermis and the deeper layers, establishing a stored electrical potential or ‘skin battery”
■ After wounding, microcurrents flow into the wound site, establishing a ‘current of injury’1 (figure 1), which stimulates cellular activity such as migration and activation of gene expression in macrophages, endothelial cells, fibroblasts and keratinocytes2, as part of normal wound healing
■ The current of injury extends up to a radius of 2-3mm around the wound3 As the wound closes, the current of injury progressively reduces
■ In chronic non-healing wounds, microcurrents are thought to dissipate and become dysfunctional or completely absent4 Application of exogenous electrical stimulation therapy is effectively restoring a normal process in wound healing to mimic the current of injury, which has become “exhausted” due to the chronicity of the wound
■ Electrical Stimulation therapy has been recognised for use internationally5. Evidence has demonstrated a reduction in pain and accelerated healing using electrical stimulation therapy
1 The Accel-Heal Solo treatment can be applied by either clinician, carer or patient
Clean the skin surrounding the wound with the alcohol wipes included
Attach two electrode pads to the patient’s skin, either side of the wound or dressing
Attach the Accel-Heal Solo device cables to the electrode pad leads by pushing the Accel-Heal cable connectors into the electrode pad cable receptors
Press and hold the button for 2 seconds to activate the Accel-Heal device. When the button is released the device will commence operation
Accel-Heal Solo delivers a pre-set programme of subsensory electrical therapy and is worn continuously for the 12-day treatment, alongside standard care such as compression therapy, as indicated. Dressings, including the electrode pad, can be changed according to clinical need
■ Accel-Heal Solo is a unique device which is very easy to use, enabling frontline use of electrical stimulation in everyday clinical practice (figure 2)
■ Accel-Heal Solo is a single-use wearable device that delivers a fixed 12-day programme of subsensory electrical stimulation therapy to hard-to-heal wounds to relieve pain and accelerate healing

■ Accel-Heal Solo is a class IIa medical device which delivers low voltage biphasic monophasic pulsed current (LVBMPC) electrical stimulation therapy to the wound - mimicking levels seen in the body’s natural systems. The device delivers repeated treatment sessions, each lasting 28 minutes and 40 seconds with a resting period repeated several times daily throughout the 12-day treatment period. Meta-analyses6,7 have shown that EST devices, like Accel-Heal Solo, that deliver a “pulsed current” stimulation are more efficacious than other types of devices
■ Accel-Heal Solo is small and discreet, about the size of a car key fob, and can be worn unobtrusively by the patient in all settings, without the need to attend specialist clinics. Accel-Heal Solo is worn continuously over the therapy period and is used alongside standard therapy to ensure wound bed preparation strategies are continued according to best practice8,9,10, and compression therapy if indicated for leg ulcers
■ The majority of patients only require one Accel-Heal Solo therapy to expedite healing of the wound. However, those patients who had a positive response to the initial treatment, which then become stalled or exacerbated, might benefit from a second application of the Accel-Heal Solo therapy
The therapy can be paused and the device and electrodes removed to enable, for instance, bathing. To pause the Accel-Heal Solo device mid-use, press and hold the button for 2 seconds
not allow the device to become
to the
for

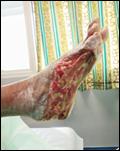
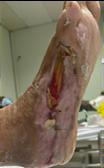
of
Day 0 - Extensive
DFU to lateral aspect right foot prior to AccelHeal therapy.
Day 9 - DFU to lateral aspect right foot following AccelHeal therapy.
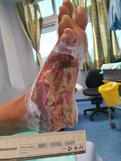

14 weeks follow ing Accel-Heal therapy - DFU to lateral aspect right foot.
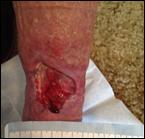
Electrical stimulation therapy (EST) is one of the most widely evidence-based therapy areas in wound management. The extensive evidence base includes nine meta-analyses, eight systematic reviews and over 35 Randomized Controlled Trials (RCTs), that describe the efficacy of EST in wound management. Accel-Heal EST has 25 published articles, including 2 double blind placebo controlled RCTs, demonstrating effectiveness in pain reduction and wound healing in hard-to-heal wounds.
■ Pain reduction has had a significant impact on many patient’s lives, with many reducing their analgesia, reporting improvements in their mobility and social lives and sleeping
■ The evidence demonstrating the positive clinical outcomes regarding pain reduction using Accel-Heal therapy is significant
■ Information has been extracted from previous studies, to include any patients/ wounds who had their pain score recorded (visual analogue score (VAS) range 0-10), prior to Accel-Heal therapy, at the end of the 12 day treatment and at the end of the study period. 71 patients from 11 studies11-12,13,14,15,16,17,18,19,20 were included in the analysis, and had been evaluated for a period of between 4.3 to 21 weeks. Many patients had pain scores >5, and were taking several types of analgesia, and suffering sleeplessness and social isolation. The data demonstrated a mean pain score of 6.81 (VAS) prior to therapy, reducing to a mean of 2.62 (61.5% reduction) after the 12-day therapy and reducing to a mean of 0.4 (94.2% reduction) by the end of the evaluation
■ A randomised gene expression analysis21 demonstrated that after 48 hours of stimulation, Accel-Heal was found to affect gene expression in human skin. In particular, Accel-Heal down-regulated 25 pro-inflammatory genes that are implicated in painful, chronic ulcers and in inflammation, supporting a mechanism behind the pain reduction that is seen in clinical practice
■ Accel-Heal has a positive impact on wound size reduction and healing, in hard-to-heal wounds. In three observational studies11,14 involving 37 nonhealing wounds, healing was achieved in 84-90% within 20 weeks. 27% (n=10) of wounds had been present for over one year prior to Accel-Heal therapy
■ To validate the healing potential even further, information has been extrapolated from several previous studies, to include any patients/ wounds who had their wound dimensions recorded prior to Accel-Heal therapy, following the treatment and at the end of the study period
■ 120 patients/ wounds from 14 studies11-20,22,24 were analysed, including complex non-healing wounds; venous leg ulcers (failed to heal despite compression therapy), DFUs, post-operative wounds and arterial ulcers. Some wounds were extensive and had been present for many years. Patients had been seen for the evaluation process for a period of between 12 days to 182 days. The analysis demonstrated a mean wound size of 17.25 cms square (range 0.04 - 133), prior to Accel-Heal therapy. Following the therapy (assessed between 12 days to 8 weeks), the mean wound size was reduced to 12.8 (reduction of 25.8%). At the end of the evaluations, the mean size was 4.97 (reduction of 71.2%)
■ Several economic studies have demonstrated the cost effectiveness of using Accel-Heal22,25-26
■ One study27 demonstrated a decrease of 74% in district nursing time over the 20 week study period, resulting in cost-efficiencies in nursing time and cost savings in dressings and bandages
■ Analysis of a previously reported RCT25 determined that wounds being treated with Accel-Heal, healed on average 3.6 weeks faster vs standard care, resulting in cost savings of £936 per patient on average
“If I hadn’t had the device put on I think I would have been struggling.” Patient in UK
“I would really and truly recommend (the treatment) to anybody who had an ulcer... the benefits... and the way you feel when you’re free of it you can’t explain to anybody.” Patient in UK
“I would recommend it to anybody... If I had to have the treatment again I would have it willingly because I couldn’t stand to have that pain again.” Patient in UK
“For a long time we were getting nowhere, I feel like because of that I lost a few years as it was a real struggle, so now I’m delighted to start improving… I have a great family around me who are all so pleased with the progress, just ask my daughter in law, she’s thrilled.”16 Patient in UK

There have been significant advances in chronic wound management treatments over the last 30 years, however, hard-to-heal wounds are an increasing problem.
Chronic wounds can also be extremely painful –between 50% and 60% of patients with a chronic wound experience persistent pain.[1,2] One major issue is that pain can make gold standard treatments, such as compression, unbearable.[3]
Electrical stimulation has been used globally in specialist clinics for more than a decade to relieve pain and accelerate healing in chronic wounds. With nine meta analyses and over 35 randomised controlled trials published, electrical stimulation is one of the most evidence-based technologies in wound management.
Electrical stimulation therapy is now available in a simple to use, single use, wearable device, enabling its more widespread use across different care settings. Accel-Heal Solo electrical stimulation wound therapy is a one-off, interventional, 12-day treatment which is applied alongside existing care to relieve pain [4] and accelerate healing [4,5]. Accel-Heal Solo improves the quality of life for the patient [5] and helps facilitate the use of compression therapy where wound pain has been an issue.[6]
To find out more about Accel-Heal Solo, please contact: customerservices@accelheal.com


Surgical wound complications can range from surgical wound dehiscence, hypergranulation, periwound maceration, scarring, medical adhesive related skin injury, seroma, and haematoma. Surgical wound complications cause significant morbidity and have significant cost implications.
“Dura est manus cirurgi, sed sans.”
Translated to ‘the hand of the surgeon is hard but healing’, is a famous quote by Walter Map. Thought provoking in its nature, because often surgery can be hard, tough and intensive on the patient. But our role as surgeons is to try to minimise damage to the tissue whilst carrying out extensive dissections, moving tissue flaps and performing skin substitute surgery and skin grafting. In other specialties such as vascular surgery procedures, lengthy dissections in multiple tissue planes are performed. Obstetric surgery has its own challenges too, including restoration of tissue to their original anatomical positions.
Although techniques in surgery are ever evolving, the basic tenets of good surgical technique remain unchanged over time. Essentially a surgeon should consider every step of his or her procedure in advance to optimise all the steps in the procedure. For most procedures we perform, we tend to write down each step of the procedure as a roadmap ahead of time. Within each of those steps of surgery it is important to optimise your technique.
Surgical wound complication (SWC) can range from the following1:
• Surgical wound dehiscence (SWD)
Surgical wound complications remain one of the most common managed wound type in clinical care and a cause of significant morbidity2
The International Surgical Wound Complications Advisory Panel (ISWCAP) has been set up to optimise care for patients with a focus on early identification of complications. The consensus report they produced is a useful guide for clinicians in their wound practice1
At the basis of these types of surgery remains an underlying set of principles of good technique that minimise wound complications.

Careful tissue handling involves:
• Minimal skin edge handing with toothed forceps thus avoiding crush and tissue damage at the edges of your surgical wounds
Judicious use of cautery devices that can cause heat damage to localised tissues and impair wound healing
Careful placement of sutures to avoid tension and strangulation and subsequent necrosis of subcutaneous tissues.
Patient groups at high risk of infection include the following:
• Patients undergoing emergency procedure
• Diabetics
• Smokers
• Immunosuppressed patients
• Long procedure times ( in excess of 2 hours)
• Undernourished or massive weight loss patients
• Older age group3
It is vital to identify patients from these groups pre-operatively to ensure you have a proactive surveillance model applied.
The National Institute of Clinical Excellence provides useful classifications for surgery4.
An incision in which no inflammation is encountered in a surgical procedure, without a break in sterile technique, and during which the respiratory, alimentary or genitourinary tracts are not entered.
Those that involve implant or prosthesis should be given antibiotic prophylaxis.
An incision through which the respiratory, alimentary, or genitourinary tract is entered under controlled conditions but with no contamination encountered.
An incision undertaken during an operation in which there is a major break in sterile technique or gross spillage from the gastrointestinal tract, or an incision in which acute, non-purulent inflammation is encountered. Open traumatic wounds that are more than 12 to 24 hours old also fall into this category.
An incision undertaken during an operation in which the viscera are perforated or when acute inflammation with pus is encountered (for example, emergency surgery for faecal peritonitis), and for traumatic wounds if treatment is delayed, there is faecal contamination, or devitalised tissue is present
Use the local antibiotic formulary and always take into account the potential adverse effects when choosing specific antibiotics for prophylaxis.
Inform patients before the operation, whenever possible, if they will need antibiotic prophylaxis, and afterwards if they have been given antibiotics during their operation.
Antibiotic prophylaxis should be given in:
• Clean surgery that involves a prosthesis or implant
• Contaminated surgery
Ask the patients to have a shower before surgery. Hair removal is no longer recommended prior to surgery. If the type of surgery indicates the need for hair removal, single use clippers are recommended in place of razors4.
Ensure that there is appropriate theatre wear for patient and staff according to your local protocols. Sterile gowns should be worn for operations, and consider double gloving, and
avoid using diathermy for surgical incisions.
The NICE guidelines recommend Leukomed Sorbact for vascular and Caesarean incisions4
Consider digital wound imaging by medical photography as part of your hospitals protocol. Don’t use topical antimicrobials for surgical wounds healing by primary intention. Report SSIs up to 30 days after surgery and 90 days after implant surgery. Monitor closely for signs of dehiscence. Red flags for urgent intervention include haemorrhaging dehiscence, burst abdomen, systemic sepsis , spreading cellulitis, dehiscence over mesh/implants or prosthesis. Ensure that baseline blood tests and wound swabs are taken.
Tip 8: Consider the Role of Negative Pressure Wound Therapy (NPWT)
NPWT has been well established in chronic wounds and there is trend towards considering it for surgical wounds in high risk patients. Particular groups that have been considered include patients with heavy exudating wounds, and wounds at high risk of infection.
Fluorescent imaging provides a useful insight into bacterial activity in the wound. It is well established in the hard-to -heal/ chronic wound groups and is gaining popularity in surgical wounds.
Along with data on surgical site infections (SSI) proactive surveillance provides information early on any surgical wound issues. This proactive approach enables the clinicians to entity problems early.
1. Optimising Prevention of Surgical Wound Complications: Detection, Diagnosis, Surveillance and Prediction. International Consensus Document 2022. Wounds International.
2. Guest J, Fuller GW, Vowden P, Vowden KR. Cohort study evaluating pressure ulcer management in clinical practice in the UK following initial presentation in the community: costs and outcomes. BMJ Open 8(7): e021769
3. Torpy J. Wound infections. JAMA. 2005;294(16):2122
4. NICE Surgical site infections: prevention and treatment. NICE guideline. Published: 11 April 2019. Available at: www.nice.org.uk/guidance/ng125
5. National Wound Care Strategy Programme 2021.
6. Guest J.F., Ayoub N., McIlwraith T., Uchegbu I., Gerrish A., Weidlich D., et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12).
7. Stryja J., Sandy-Hodgetts K., Collier M., Moser C., Ousey K., Probst S., et al. Surgical Site Infection: Prevention and Management across Health-Care Sectors. Journal of Wound Care. 2020;29(Sup 2b):S1-S72. Available at: https://ewma.conference2web.com/#resources/384542
8. Sandy-Hodgetts K., Carville K., Leslie G.D. Surgical wound dehiscence: a conceptual framework for patient assessment. Journal of Wound Care. 2018;27(3):119-26.
9. Gray T.A., Rhodes S., Atkinson R.A., Rothwell K., Wilson P., Dumville J.C., et al. Opportunities for better value wound care: a multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open. 2018;8(3).
10. World Health Organisation. Global Action Plan on Antimicrobial Resistance. 2015. 6. Lipsky B.A., Dryden M., Gottrup F., Nathwani D., Seaton R.A., Stryja J. Antimicrobial stewardship in wound care: a Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. Journal of Antimicrobial Chemotherapy. 2016;71(11):3026-35.
11. Sandy-Hodgetts, K. Best Practice Statement: Antimicrobial stewardship strategies for wound management. Wounds UK, 2020 London. Wounds International.
This Masterclass Guide is a concise overview of sterile single use bacterial binding wound dressings, for incorporation into your clinical practice.
Surgical site infections (SSI), are the most frequently reported healthcare acquired infection and carry the heaviest financial impact for healthcare systems.
Conventionally, wound infections are treated with antimicrobials. However, with the escalating threat of antimicrobial resistance, an alternative approach needs to be adopted.
What Is Leukomed® Sorbact®?
■ Leukomed® Sorbact® is a sterile single-use dressing developed for use in closed surgical wounds. It offers a dual effect of binding bacteria at the wound bed and protecting the wound from external contamination
■ A transparent, showerproof, and easily applied polyurethane film adhesive outer layer protects and keeps the wound moist, and covers an absorbent pad with a green contact layer coated with dialkylcarbamoyl chloride (DACC), (a fatty acid derivative), which irreversibly binds the bacteria (Figure 1)
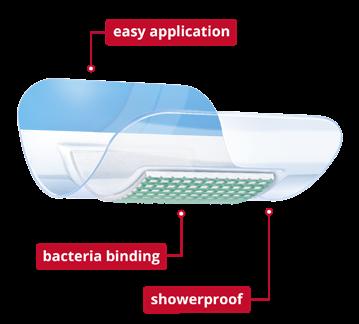
■ The dressing utilizes a unique technology by irreversibly binding the bacteria and is proven to prevent surgical site infections (SSI)
■ Leukomed® Sorbact® is recommended for usage to prevent SSI by the National Institute for Clinical Excellence (NICE)1
■ Reducing the bioburden of a surgical wound will facilitate the healing process
■ These dressings work on the principle that two substances with hydrophobic properties will bind together when introduced in a moist environment (hydrophobic interaction)
■ The dressing comprises of an absorbent non-woven wound contact pad, and an outer, transparent, adhesive polyurethane film
■ The pad is made of a white viscose polypropylene and polyester mesh, coated with DACC. Upon contact, the bacteria are irreversibly bound with the DACC coated layer, their growth is inhibited and they can be removed from the wound area when the dressing is changed
■ Since the DACC method does not kill the bacteria, there is no release of endotoxins or cell debris. The purely physical method of binding and removing the bacteria also minimizes the risk of development of resistance
1 Prepare the wound according to local clinical practice. Ensure that the surrounding skin is clean and dry
2 Select an appropriate dressing size for the wound
3 Remove the dressing from the pouch using an aseptic technique
4 Remove the protective film from the wound contact side of the dressing and apply the dressing. Ensure that the green wound contact layer comes into direct contact with the complete wound surface. Do not stretch the film during application
5 Press the borders to the surrounding skin. Carefully remove the application film, using the blue handling strip. Slowly pull the application film backwards, pulling parallel to the skin to avoid it lifting
6 The dressing change frequency depends on exudate levels and the overall condition of the wound and surrounding skin. Should the clinical condition allow, the dressing can be left in place for up to 7 days
7 For gentle removal, stretch the dressing to release adhesion. Pull one edge of the dressing parallel to the skin. Do not pull up or backwards.
8 Avoid lifting the dressing for inspection
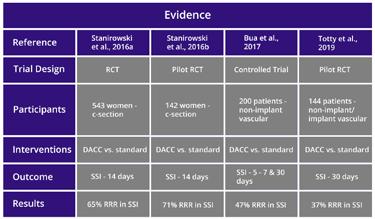




■
■
■
in
relative risk
in
in
■ SSI is a common complication following caesarean section, and the procedure has higher rates of this than other surgeries, affecting 1.8 - 9.2% of patients undergoing this procedure2, (Figure 2). The consequences are potentially devastating for the individual and costly for hospitals and healthcare systems
■ The success in use of Leukomed® Sorbact® dressings in the management of surgical wounds of patients following this procedure, to prevent SSI, is well evidenced1
■ Stanirowski et al. conducted a randomized, single-blinded controlled study which assessed the efficacy of Leukomed® Sorbact® in preventing SSI in women after a caesarean section. The participants were randomly assigned to two groups, one given standard surgical dressings and the other group given Leukomed® Sorbact® dressings2
■ Stanirowski notes a decreased rate of SSI in the group given Leukomed® Sorbact® dressings2 (Figure 2)
■ Bua et al. report on a prospective, nonrandomized comparative study in a single vascular surgery center, involving two groups of 100 participants, one which was treated with Leukomed® Sorbact® dressings, and the other group given the standard inert surgical dressings normally used by the clinicians of the facility, concluding:
■ “DACC coated dressings were associated with a significant reduction in SSI rates in the early post-operative period.”3 (Figure 2)
■ “Dialkylcarbamoyl chloride (DACC)coated dressings in the management and prevention of wound infection: a systematic review’ considers 17 studies involving 3408 patients, to conclude that: “DACC-coating of dressings shows promise in both the prevention and treatment of wound infections... the available evidence that is presented is in support of DACC-coated dressings, and such promise does allow for the undertaking of further high-quality research into their clinical and costeffectiveness.”4
■ Stanirowski notes reduced costs in treatment of the study group treated with Leukomed® Sorbact®, due to reduced need for further treatment resulting from SSI2 (Figure 2)
■ The NICE (The National Institute for Health and Care Excellence) guidance for Leukomed® Sorbact® further supports the cost effectiveness, efficacy and safety in use of Leukomed® Sorbact® dressings1
■ “Hydrophobic dressing application seemed to result in a decreased rate of SSI and a considerably milder course, with no need for systemic antibiotic therapy and hospital readmissions.”
“Total cost of SSI treatment was lower… and was a result of ambulatory visits only.”2
■ “Leukomed Sorbact is cost saving across a wide range of SSI costs, device costs, comparator costs and relative risk reduction.”1
■ “DACC coated hydrophobic dressings have effects in preventing SSI in a number of different patient groups and may have a significant role in future surgical wound management.”3


SSIs are the third most commonly reported type of healthcare-acquired infection and the most costly¹. They place a significant impact on patient welfare² as well as presenting a heavy financial burden for the NHS³.

(SSI) in
with low to
section and vascular surgery.
to https://www.nice.org.uk/guidance/mtg55
Diabetic Foot Ulcers (DFUs) are common and are a major source of disability, distress, and cost. DFUs are difficult to heal because of poor vascularity and impaired sensation resulting from sensory neuropathy. There are around 7,000 lower limb amputations in diabetes patients in England each year, and the likelihood that someone with diabetes will have a leg, foot or toe amputation is around 23 times that of a person without diabetes.
In recent years there is great interest in the use of autologous platelet rich products for use in DFUs. Topical growth factor products are typically used as adjuvant treatments along with the standard care for treatment of diabetic foot ulceration with successful results. Endoret® PRGF® (Plasma Rich Growth Factors) uses advanced biomedical technology to stimulate tissue regeneration with the use of autologous proteins found in the blood.
The Endoret® PRGF® technique involves a simple bedside procedure to obtain a platelet concentrated fibrin membrane rich in autologous growth factors, which acts as a biological membrane. Endoret® PRGF® preparation was shown to have higher levels of most growth factors which help in biological wound healing. This preparation has demonstrated its effectiveness in multiple medical fields including dentistry, oral implantology, opthalmology and orthopedics,
among others.
To improve wound healing and to accelerate the epithelization of chronic wounds, we applied the Endoret® technique on diabetic patients with chronic, non-healing DFUs to test PRGF clot membrane therapy.

The Endoret® technique consists of a single treatment. Venous blood was obtained and collected using the Endoret® PRGF® protocol in 9ml sterile tubes containing 3.8% (wt/v) Sodium Citrate (SC). Using Endoret® patented technology, the blood was centrifuged for 8 minutes at room temperature using the Endoret® PRGF® system centrifuge to obtain PRGF. The PRGF was drawn off in 2 fractions, labelled F1 and F2. F1 was injected around the wound margins. Two millilitres of F2, which contains the optimal concentration of platelets (2-3x) isolated above the erythrocytes and leucocytes, was drawn up using the Plasma Transfer Device (PTD). F2 was then activated using Calcium Chloride (CC). Upon completion of the Endoret® protocol, a fibrin clot was formed and placed over the bed of the ulcer, and the remaining F2 was infiltrated around the edges of the wound. Adequate dressing was secured, and the patient kept non-weight-bearing.
Subsequent follow-ups were done in the clinic at 2 weekly intervals for examination of the wound, dressing and medical photography by a trained nurse. In all cases, the entire procedure took under 1 hour. Patients were followed up until the end point was achieved, which was defined as full epithelization of ulcer without drainage.
Mr Noman Niazi
All patients achieved full epithelization of the ulcers with no adverse events or complications observed throughout the treatment.
PRGF therapy has been successfully used to promote tissue regeneration and has been employed in the treatment of multiple musculoskeletal disorders and regeneration of tissue healing. In recent years, several studies have reported the effects of different plateletrich products in diabetic ulcers, obtaining successful results in ulcer healing. One Randomised Control Trial (RCT) of autologous platelet gel reported benefit in time to complete ulcer closure at 12 weeks in comparison with standard care. A recent multicentric RCT showed clinically and statistically significant benefit with the application of autologous immune cell, fibrin, and platelet patches for DFUs.
Endoret® PRGF® offers many distinct advantages over traditional platelet rich plasma (PRP) therapy by creating a more rapid and natural healing response. Hundreds of endogenous proteins stimulate the tissue repair processes, which include new blood vessel formation, cell mobilization and cell proliferation. It also provides a safe and excellent vehicle for local growth factor delivery, functioning as a provisional matrix that supports the ingrowth of neovasculature and the migration of cells into the ulcer dead space, regenerating surrounding soft tissues. This single treatment is a useful adjuvant to skin dressings for DFUs to improve outcome and decrease serious complications. Successful treatment of DFUs may decrease further diabetic complications including amputations and ultimately mortality.
“Successful treatment of DFUs may decrease further diabetic complications including amputations and ultimately mortality.”The Role of Topical Autologous Plasma Rich Membranes in Healing Diabetic Foot Ulcers


When it comes to consistency in quality, care and savings look no further than Kliniderm foam silicone.
A trusted choice whatever your supply route – savings of up to 47%*.

This article provides a concise overview of the role of oxygen therapy in your clinical wound practice. Oxygen is required at almost every step of the wound healing process1. Oxygen is an essential building block of cells and tissues, required for synthesis of energy, proteins, and collagen. If these building blocks are not present, tissue building, and wound healing simply cannot occur9. Oxygen is also involved in many wound healing processes, such as oxidative killing of bacteria and angiogenesis9.
Chronic and hard to heal wounds are a silent epidemic, affecting a large percentage of the global population1. 1-2 of every 100 adults will suffer from a chronic wound in their lifetime, many suffering from multiple wounds and recurrences, and these rates will only increase with our ageing population2. In developed countries, wound care accounts for ~3% of total health care expenditures3. This translates to annual estimated costs of more than 97 billion dollars in the United States3 and 5.3 billion pounds in the UK4. The most effective way to reduce these costs is simply to heal wounds with chronicity that are considered ‘non-healing’.
Yet, in practice, healing these wounds is far from simple. Despite the enormous range of readily available bandages, ointments, antimicrobial solutions, and advanced therapies targeted to wounds, the current standard of care heals less than 40% of chronic wounds by 12 weeks5. The remaining wounds continue to be a burden to both the patients and the healthcare system.
Diabetic ulcers, vascular ulcers (venous or arterial), and pressure injuries are all chronic wounds. The pathologies underlying chronic wounds can differ widely. However, common shared features include prolonged or excessive inflammation, persistent infections, and the inability to respond to reparative stimuli6,7
Adults with vascular disease and/or diabetes are at highest risk for chronic leg and foot wounds.
The ischemic (reduced tissue perfusion) and/ or hypoxic lower limb conditions which result
from these conditions reduces availability of both oxygen and nutrients, making these wounds especially hard to heal. These wounds last on average 12 to 13 months, but this varies widely; many will remain open for years or never heal6,7, and up to 30% of Diabetic Foot Ulcers (DFUs) go on to amputation8. Even when they do heal, wounds recur in 60-70% of patients, decrease quality of life, and are a significant cause of morbidity6,7
Oxygen is required at almost every step of the wound healing process1. Oxygen is an essential building block of cells and tissues, required for synthesis of energy, proteins, and collagen. If these building blocks are not present, tissue building, and wound healing simply cannot occur9. Oxygen is also involved in many wound healing processes, such as oxidative killing of bacteria and angiogenesis9. Yet, both wound perfusion and blood oxygen levels are frequently insufficient in patients with chronic wounds due to poor circulation, vascular disruption, and vasoconstriction, thereby reducing the wound’s capacity to heal.Novel wound care therapies that specifically address these underlying issues are sorely needed. Fortunately, there is a growing body of evidence to support the benefits of supplementary oxygen therapy on wound healing10-12.
When physiological constraints hinder adequate oxygenation, various methods can be used to direct exogenous oxygen to the wound in a therapeutic context. Skin wounds can receive oxygen from the blood stream via perfusion and from oxygen uptake through the skin13. Raising oxygen concentrations can, by itself, trigger healing responses through the mechanisms
outlined earlier.
Severe impairments in lower limb vascular networks, suffered by many patients with wounds, often necessitate surgical efforts to improve these oxygen delivery networks and prevent amputation14,15
Direct repair of perfusion can therefore have a profound effect on restoration of tissue oxygenation. Revascularization via arterial bypass surgery, venous ablation, angioplasty, and stenting are methods that attempt to correct the lower limb large vessel network15. When successful, the benefits are clear; limbs on the path to amputation are often saved by these procedures14,16. However, surgical interventions improve flow primarily in larger arteries; they often cannot overcome deficiencies in the microvasculature, which is a root cause of poor tissue oxygenation in hard to heal wounds17,18.
Therefore, many wounds remain slow to heal after these procedures and wound recurrence is very common17,19. Benefits of adjunctive oxygen therapy on wound healing in this group have been reported20
Improved wound oxygenation can be achieved with the use of supplementary oxygen. This can be delivered either systemically or topically, depending on your patient’s needs.
In some patients, systemic Hyperbaric Oxygen Therapy (HBOT) can be very effective at increasing wound oxygen to above physiological levels, resulting in a pharmacologic dose21. As these pharmacological doses are systemic, i.e., not directed at the wound, effectiveness of hyperbaric oxygen is somewhat dependent on adequate blood perfusion. The vascular system must direct and deliver the pharmacologic oxygen levels to the wound. This is hampered in the large percentage of patients with wounds
in which perfusion is compromised by peripheral artery disease or chronic venous insufficiency. Still, when adequate arterial blood flow to the wound is present, adjunctive hyperbaric oxygen in patients with lower limb chronic wounds has been shown to elevate wound oxygen levels21, improve wound healing rates22, and reduce limb amputation rates23. In addition to the elevated oxygen levels, there is evidence to suggest that hyperbaric therapy may increase angiogenesis and antioxidant response24 and decrease inflammation25. Despite these reported benefits, practical limitations and adverse effects have hindered the global adoption of hyperbaric oxygen as a wound therapy.
Systemic HBOT is costly, not widely available, and requires patients to be confined to a full body chamber for at least 90 minutes a day, 5 days a week for the duration of the treatment26 These lifestyle-limiting chambers are not portable, and therefore cannot be used for supplying continuous oxygen therapy to the patient in an outpatient setting. Furthermore, the number of hyperbaric sessions a patient can tolerate is limited; middle ear barotrauma has been observed in two-thirds of patients after just seven days of systemic hyperbaric treatment27,28. Consequently, hyperbaric oxygen chamber usage is limited to very short periods of the week (approximately 5%)26, which in turn limits the ability to raise oxygen levels in wounds for any sustained period.
The pre-clinical and clinical studies provide ample evidence to support Topical Oxygen Therapy (TOT) use. Pre-clinical models involving excisional dermal wounds in pigs exposed to TOT had increased wound tissue pO2 (partial pressure of oxygen); within 4 minutes oxygen had diffused into the wound tissue and elevated the local pO2 from 5 mmHg to over 40 mmHg (a 10-fold increase in pO2 after 4 minutes)26. Low pressure topical oxygen, in contrast to HBOT, can diffuse locally at the wound bed, deep into the tissue and elevate
“The pre-clinical and clinical studies provide ample evidence to support Topical Oxygen Therapy (TOT) use.”
“TOT can be delivered either intermittent or continuous. The main difference is intermittent systems tend to be pressurised systems whereas continuous TOT are portable low flow systems. These systems are particularly relevant today, because they are home care based, offering an alternative for wound patients particularly during COVID-19.”
local tissue partial pressures with repeated treatments accelerating wound closure. TOT induces vascular endothelial growth factor expression and improves closure of chronic wounds27. Oxygen levels have also been shown in both animal and human studies to significantly increase growth factors related to the formation of angiogenesis; Vascular Endothelial Growth Factor (VEGF), Basic Fibroblast Growth Factor (FgF-2) and PlateletDerived Growth Factor (PDGF)27. Several controlled TOT studies in humans have showed significantly lower reoccurrence rates at 36 months post healing in the TOT group28,29 (better interlinked collagen and resultantly more patent tissue). TOT on the hind limb wounds of rats under ischemic conditions had shorter healing times, more accumulation of collagen fibers, and many more new vessels compared to the control group30. The results of the experiment showed that TOT therapy improved ischemic wound healing.
TOT can be delivered either intermittent or continuous. The main difference is that intermittent systems tend to be pressurised systems, whereas continuous TOT are portable low flow systems. These systems are particularly relevant today, because they are home care based, offering an alternative for wound patients particularly during COVID-19.
TOT improved healing of even advanced (Texas Grade III) DFUs31, or wounds that had been open for up to 88 weeks prior to topical oxygen therapy32. A recent Randomized Controlled Trial (RCT) with 54% of patients over age 65, demonstrated statistically significant healing rates in the continuous diffusion TOT treated patients33. Over 52% of those patients healed within the 12-week therapy period, representing a 71% greater healing rate and 73% greater reduction in wound size compared to the control group33. Kauffman et al., (2018) reported the results of a large trial in chronic ulcers of mixed aetiology34. The authors noted
an optimum treatment effect if the continuous diffusion device was used for >25 days, including an 83% reduction in wound area and a 47% wound closure rate in venous leg ulcers, and a 74% reduction and 57% wound closure rate in arterial foot ulcers. The effects of the continuous delivery of oxygen to the wound have also been examined mechanistically: Hunter et al., (2019) used 16S rDNA sequencing to examine the effect of topical oxygen on chronic wound microbiomes. The authors reported a significant shift in bacterial flora from anaerobic to aerobic (oxygen utilizing) genera after 2 weeks of continuous treatment with the TOT system35. The authors postulated based on their results that topical oxygen can promote healing via disruption of the bacterial biofilms that form in chronic wounds35. A sham-controlled, double-blind randomized controlled trial demonstrated that, at both 12 weeks and at 12 months, adjunctive cyclical pressurized TOT therapy was superior in healing chronic DFUs compared with optimal SOC alone36 with a fourand-a-half-fold increased likelihood of healing achieved at 12 weeks. Clinically, the durability of healing as measured by index ulcer recurrence at 12 months was sixfold better than that in the sham group36. Home based cyclical pressurized topical wound oxygen therapy, when used with or without other adjunctive treatments, is associated with significantly reduced frequency of wound-related hospitalization and amputation for patients afflicted with DFU37 That study found 8.9 times greater likelihood of hospitalizations in the non-oxygen therapy arm compared to oxygen, and similarly 4.9 times greater likelihood of amputation in the nonoxygen arm compared to the oxygen therapy arm37
We know oxygen plays a significant role during every phase of wound healing from angiogenesis and revascularization to cell membrane and energy production, it promotes growth factor
Optimizingsignaling transduction, collagen synthesis, cell proliferation and re-epithelization, and it even is involved in antibacterial activities and bacterial biofilm. Just like wound care products and dressings, there are various options available. Knowing your patient, their needs, available resources, and support level, you can confidently select an oxygen delivery system that will enhance your wound care outcomes.
1. Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen 2009;17:1-18.
2. Gottrup F. A specialized wound-healing centre concept: importance of a multidisciplinary department structure and urgical treatment facilities in the treatment of chronic wounds.
American journal of surgery 2004;187:38S-43S.
3. Nussbaum SR, Carter MJ, Fife CE, et al. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. 2018;21(1):27-32.
4. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015 5(12):e009283.
5. Fife CEE, K.A; Carter, M.J. Publicly Reported Wound Healing Rates: The Fantasy and the Reality. Advances in wound care. 2018; 7(17).
6. Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle). 2015;4(9):560-582.
7. Richmond NA, Maderal AD, Vivas AC. Evidence-based management of common chronic lower extremity ulcers. Dermatol Ther. 2013;26(3):187-196.
8. Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care. 2004;27(9):2129-2134.
9. Gottrup F, Hunt TK, Hopf HW. Role of oxygen in wound healing and infection. Journal of wound technology 2010; 9: 6-10
10. Shivshankar T, Singh T, Golledge J. Topical oxygen therapy for diabetes related foot ulcers: A systematic review and meta analysis. Diabetic Medicine 2021 38: e14585.
11. Tawfick WA, Sultan S. Technical and clinical outcome of topical wound oxygen in comparison to conventional compression dressings in the management of refractory nonhealing venous ulcers. Vasc Endovascular Surg. 2013 47(1): 30-37.
12. Eggleton PB, Smerdon, G.R. Safety and efficacy of hyperbaric oxygen therapy in chronic wound management: current evidence. Chronic Wound Care Management and Research. 2015 2(12).
13. Stucker M, Struk A Altmeyer P et al The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and Epidermis Journal of Physiology2002 538(3):985–994
14. Almasri J, Adusumalli J, Asi N, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J Vasc Surg. 2018;68(2):624-633.
15. Conte MS. Diabetic revascularization: endovascular versus open bypass--do we have the answer? Semin Vasc Surg. 2012;25(2):108-14.
16. Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-Year Trends in Lower Limb Amputation, Revascularization, and Preventive Measures Among Medicare Patients. JAMA Surg. 2015;150(1):84–86.
17. Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes ObesMetab. 2015;17(5):435-44.
18. Wollina U, Abdel-Naser MB, Mani R. A review of the microcirculation in skin in patients with chronic venous insufficiency: the problem and the evidence available for therapeutic options. Int J Low Extrem Wounds. 2006;5(3):169-80.
19. Stoekenbroek RM, Santema TB, Legemate DA, et al. Hyperbaric oxygen for the treatment of diabetic foot ulcers: a systematic review. Eur J VascEndovasc Surg. 2014;47(6):647-55.
20. Rhodes JM, Gloviczki P, Canton LG, et al. Factors affecting clinical outcome following endoscopic perforator vein ablation. Am J Surg. 1998;176(2):162-7.
21. Smith BM, Desvigne LD, Slade JB et al. Transcutaneous oxygen measurements predict healing of leg wounds with hyperbaric therapy. Wound Repair Regen. 1996;4(2):224-229.
22. Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25(6):513-518.
Variables to consider while deciding which oxygen delivery system to choose from include: activity level, wound impacting factors; patient comfort levels, economic impact on your patient; geographic location, capable participation level; compliance/ adherence to therapy, number of wounds and severity; presence of active infection, and dressing compatibility.
23. Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338-1343.
24. Sureda A, Batle JM, Martorell M, et al. Antioxidant Response of Chronic Wounds to Hyperbaric Oxygen Therapy. PLoS One. 2016;11(9):e0163371.
25. Thom SR. Effects of hyperoxia on neutrophil adhesion. Undersea Hyperb Med. 2004;31(1):123-131.
26. Winfield B. Topical Oxygen and Hyperbaric Oxygen Therapy Use and Healing Rates in Diabetic Foot Ulcers. Wounds. 2014;26:E39-E47. Fries RB, Wallace WA, Roy S, Kuppusamy P, Bergdall V, Gordillo GM, Melvin WS, Sen CK. Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res. 2005 Nov 11;579(1-2):17281. doi: 10.1016/j.mrfmmm.2005.02.023. Epub 2005 Aug 18. PMID: 16105672.
27. Blanshard J, Toma A, Bryson P, et al. Middle ear barotrauma in patients undergoing hyperbaric oxygen therapy. Clin Otolaryngol Allied Sci. 1996;21(5):400-403.Gordillo GM, Roy S, Khanna S, Schlanger R, Khandelwal S, Phillips G, Sen CK. Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol. 2008 Aug;35(8):957-64. doi: 10.1111/j.14401681.2008.04934.x. Epub 2008 Apr 21. PMID: 18430064; PMCID: PMC2574754.
28. Karahatay S, Yilmaz YF, Birkent H, et al. Middle ear barotrauma with hyperbaric oxygen therapy: incidence and the predictive value of the nine-step inflation/deflation test and otoscopy. Ear Nose Throat J. 2008;87(12):684-688. Blackman E, Moore C, Hyatt J, Railton R, Frye C. Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage. 2010 Jun;56(6):24-31. PMID: 20567051.
29. Tawfick W, Sultan S. Does topical wound oxygen (TWO2) offer an improved outcome over conventional compression dressings (CCD) in the management of refractory venous ulcers (RVU)? A parallel observational comparative study. Eur J VascEndovasc Surg. 2009 Jul;38(1):125-32. doi: 10.1016/j.ejvs.2009.03.027. Epub 2009 May 22. PMID: 19464933.
30. Rao C, Xiao L, Liu H, et al. Effects of topical oxygen therapy on ischemic wound healing. J Phys Ther Sci. 2016;28(1):118-123. doi:10.1589/jpts.28.118
31. Yu J, Lu S, McLaren AM, Perry JA, Cross KM. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen. 2016;24(6):1066-1072.
32. Hayes PD, Alzuhir N, Curran G, Loftus IM. Topical oxygen therapy promotes the healing of chronic diabetic foot ulcers: a pilot study. J Wound Care. 2017;26(11):652-660.
33. Serena TE, Bullock NM, Cole W et al. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled trial. J Wound Care 2021; 30: Suppl.5 S7-14.
34. Kaufman H, Gurevich M, Tamir E, Keren E, Alexander L, Hayes P. Topical oxygen therapy stimulates healing in difficult, chronic wounds: a tertiary centre experience. J Wound Care. 2018;27(7):426-433.
35. Hunter P, Greco E, Cross K, Perry J. Topical Oxygen Therapy Shifts Microbiome Dynamics in Chronic Diabetic Foot Ulcers. Wounds. 2020;32(3):81-85.
36. Frykberg RG, Franks PJ, Edmonds M, Brantley JN, Téot L, Wild T, Garoufalis MG, Lee AM, Thompson JA, Reach G, Dove CR, Lachgar K, Grotemeyer D, Renton SC; TWO2 Study Group. A Multinational, Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy of Cyclical Topical Wound Oxygen (TWO2) Therapy in the Treatment of Chronic Diabetic Foot Ulcers: The TWO2 Study. Diabetes Care. 2020 Mar;43(3):616-624. doi: 10.2337/dc19-0476. Epub 2019 Oct 16. PMID: 31619393.
37. Yellin JI, Gaebler JA, Zhou FF, Niecko T, Novins O, Ockert A, Krzynowek D, Garoufalis MG, Lee AM, Frykberg RG. Reduced Hospitalizations and Amputations in Patients with Diabetic Foot Ulcers Treated with Cyclical Pressurized Topical Wound Oxygen Therapy: Real-World Outcomes. Adv Wound Care (New Rochelle). 2021 Dec 6. doi: 10.1089/ wound.2021.0118. Epub ahead of print. PMID: 34714167.
“Knowing your patient, their needs, available resources, and support level you can confidently select an oxygen delivery system that will enhance your wound care outcomes.”

Continuous topical oxygen therapy (cTOT) is a modality of wound treatment involving the application of pure, humidified oxygen to the wound area to stimulate healing, by means of a specialized device.
This Masterclass Guide explores, in particular, NATROX
. It provides a concise but detailed summary of the specifications, key points, suitability of wound types and patients, and practical application of this device.
■ The NATROX® O2 device (figure 1), is a small, portable unit which fits in the hand (figure 2). It consists of the NATROX® Oxygen Generator (OG), the NATROX® Oxygen Delivery System (ODS), and the battery (figure 3).
■ The OG is battery-operated and reusable. It is about the size and weight of a mobile phone, weighing 107g (3.7 oz)
■ The ODS is a sterile, single-use interface with a “wheel” shape to allow free passage of exudate into the secondary dressing while optimizing oxygen flow
■ The function of this device is to produce concentrated oxygen from the surrounding air, which passes through a flexible tube directly to wounds in need of advanced care. The device uses the natural properties of oxygen to stimulate and improve wound healing by delivering continuous, pure, humidified oxygen directly to the wound bed
■ Small, portable, lightweight, and rechargeable, giving patients complete freedom of movement. It can be applied across any healthcare setting, including in cases where treatment at home is more appropriate or convenient, or when self-administered by a patient
■ Can be used with other advanced wound therapies and secondary dressings
■ The system is supplied with 2 rechargeable, interchangable batteries
1 Prior to application, remove all old dressings and clean the wound using an aseptic technique, in accordance with local policies
2 Remove the ODS directly from packaging using an aseptic technique and apply directly to the wound bed, with the white side down
3 Remember to consider the position for the tubing for optimum patient comfort. The tubing can be positioned in any direction and, if necessary, can be secured with tape
4 Cover the ODS with a suitable absorbent dressing, sealing it to capture the oxygen produced by the device. You can use a dressing with an absorbent border, a bandage, or secure the edge with adhesive tape
5 After the dressing is secured, slide a fully charged battery into the device. There is no on/ off switch. Sliding the battery into the device will automatically start the device. Within 30 seconds, you will see the green light flashing which indicates that the oxygen is flowing
6 Connect the ODS to the OG using the twist and lock connector, being careful not to overtighten
7 The ODS should be changed whenever the secondary dressing is changed. However, it will need to be changed at least once a week
8 Fit the OG into the holster provided, or where the user feels most comfortable. The tubing must not be bent or distorted
9 The NATROX® O2 battery should be changed every 24 hours to ensure an uninterrupted supply of oxygen. Plug the battery into the charger (included with the kit) and leave charging all day. A flashing amber light indicates the battery is charging and a solid amber light means it is fully charged
10 To change the battery, slide the used battery out of the NATROX® O2 generator. Then slide the newly charged battery into the device, making sure it clicks into place. Charge the used battery to ensure it is ready for the following day

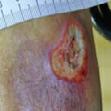
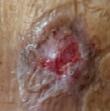
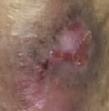



Oxygen levels have been shown to be depleted in certain wound types, such as diabetic foot ulcers, pressure, venous and arterial ulcers3. Oxygen is a vital component of the wound healing cycle, and the demand for oxygen exponentially increases in the healing wound; therefore, supplemental topical oxygen can assist in this process as well as promoting angiogenesis.
Studies show patients with a chronic wound have a 71% greater chance of healing using NATROX® O2 than with standard of care alone5
■ “TO treatment is associated with induction in VEGF expression in the wound edge tissue and improvement in wound closure outcome. Approaches to topically oxygenate exposed dermal wound tissue warrant serious interest.”3
■ Topical oxygen therapy is associated with pain relief and reduction in the need for pharmacological analgesia4
■ In patients with painful ulcers, 76% felt rapid pain relief, 69% stopped taking opioids, and 53% became pain free4
■ Topical oxygen therapy results in a statistically significant improvement in healing rates for DFUs graded IDSA 1 or 25
■ “NATROX O2 is associated with a higher rate of complete wound healing in DFU when added to standard care.”5
Yu et al. conducted a trial using NATROX® Oxygen Wound Therapy to treat DFUs, involving 20 participants6:
■ The therapy was performed weekly, for a period of 8 weeks
■ The wound size was significantly reduced compared with baseline in the NATROX® O2 group
■ Prior to the trial, the DFUs were present without healing for a mean duration of 76 weeks
■ In both groups, all grade 1 ulcers healed with complete wound closure
■ In the NATROX® O2 group, all grade 2 ulcers healed compared with none in the control group
■ In the NATROX® O2 group, 50% of grade 3 ulcers healed, compared with none in the control group
Kaufman et al. report the following results of an observational study using NATROX® O2 to treat a variety of cases, including venous leg ulcers, arterial ulcers, DFUs, trauma, burns, post-operative wounds and pressure ulcers7:
■ Adherence with the treatment was 88% and there was a mean reduction in wound area of 7% per week
■ For patients treated for more than 25 days, 31 of 65 wounds healed completely
■ There was no statistically significant differences between the groups, but non-healing ulcers tended to be larger, with a longer duration
This technology is more cost-effective and convenient compared to other methods, for reasons including:
■ Suitable for self-care in a home setting, meaning hospitalisation may not be necessary at all
■ Low cost of device
■ Small, light, wearable
■ Minimal training necessary
■ Ease of use means the therapy can be performed quickly and efficiently
Can help alleviate the growing cost of chronic wounds, as evidenced by:
■ Meta-analysis suggesting cTOT significantly increases the likelihood of ulcer healing compared to controls18
■ 85% of patients of patients treated with NATROX® O2 remained healed at 1 year19
■ Amputation rates being significantly lower when cTOT is used in conjunction with standard of care20
3 studies, a randomised controlled trial and 2 observational studies – a total of 172 adults in secondary care. They show that NATROX® O₂ effectively treats a range of chronic wounds, and is more effective than standard of care in people with grade 2 and grade 3 diabetic foot ulcers.
Kaufman et al. study: “Important real-world data from consecutive cases.”
Yu et al. study: “This was a high-quality study with well-defined interventions and patient groups. Ulcers were graded with the widely used University of Texas diabetic foot ulcer classification.”






Poor patient and service outcomes have a long association with Pressure Ulcers (PU). Despite treatment and preventative advances the development of a PU remains a significant risk during surgery, due mainly to patients being immobile for a sustained period of time, although certain co-morbidities also elevate the risk. There is a need for clinicians to have a heightened awareness of the complex interplay of factors that can lead to the development of a PU related to having a surgical procedure. In practice, traditional risk assessment scales often do not work. This means that there is scope for the use of tools and devices that measure PU risk in real time during surgery. There should be a greater concentration of effort in this area from a research perspective.
ressure ulcers (PU) have a long established relationship with poor patient outcomes1. Despite technological advances and preventative interventions, PU continue to emerge across different healthcare settings, raising service costs, increasing nurse time spent caring for a problem that is preventable, worsening patient outcomes and in some cases increasing mortality2. Patients undergoing surgical procedures are at high risk for developing a PU3. The reason for this is because during surgery patients are immobile, positioned on a generally hard surface area and unable to feel the discomfort caused by both the pressure and shear forces that accompany a surgical procedure4. In the past two decades, there have been a number of efforts to determine the link between surgery and PU. For example, a prospective cohort study observed that a total of 21.2% (n=44) of patients developed a total of 70 PUs within the first two days following a surgical procedure5. Similarly, in a longitudinal study a total of 12.7% (n=13) of patients developed a PU directly following a surgical procedure6. Notable about both these studies is that patients were free of any signs of a PU prior to their surgical procedure. Therefore, it is very likely that the development of a post-operative PU is linked directly to the intraoperative (surgical) period. Supporting this, in a prospective cohort study, Schoonhoven et al., (2006) found that surgery in the coming week was an independent predictor for PU development (grade 2 or higher), within an acute hospital setting consisting of both medical and surgical inpatients (OR 4.0 CI 2.5-6.5)7. This evidence points the finger at surgery placing patients at high risk of PU development.
Recognising the risk that surgery places patients at, it is important to consider the specific risk factors associated with PU development subsequent to a surgical procedure. Doing this will heighten clinician awareness about how to best prevent early stage PU in the immediate aftermath of a surgical procedure. However, the evidence indicates that PU prevention in surgical patients is not as straight forward as it may seem. For example, a retrospective study examining predisposing factors for PU development during surgery found an increased risk for patients placed in the supine position, with a longer operation duration (≥4 hours), and patients
Ms Hannah Wilson PhD Scholar, Skin Wounds and Trauma Research Centre; RCSI University of Medicine and Health Sciences Dublin, Ireland Prof Declan Patton Director of Nursing and Midwifery Research, School of Nursing and Midwifery; Deputy Director of the Skin Wounds and Trauma Research Centre; RCSI University of Medicine and Health Sciences Dublin, Ireland Prof Zena Moore Head of School of Nursing and Midwifery; Director of the Skin Wounds and Trauma Research Centre; RCSI University of Medicine and Health Sciences Dublin, Ireland Dr Pinar Avsar Lecturer, School of Nursing and Midwifery; Lead Researcher, Skin Wounds and Trauma Research Centre; RCSI University of Medicine and Health Sciences Dublin, Ireland Wound Masterclasswith comorbidities affecting tissue perfusion, such as patients with a history of diabetes or hypertension, also heightening the risk of PU development 8. Similarly, in a multiple logistic regression analysis, risk factors for intraoperative PU development were investigated, whereby an increase of 96% was found for surgery lasting one hour or longer (OR = 1.96, p<.001), and a 2.1 fold increase was observed for patients in the prone position versus the horizontal position (OR = 3.10, p=.01)9. This very brief snapshot of the evidence demonstrates the complex interplay of factors that can effect the development of a PU during a surgical procedure. Throughout the literature, it is apparent that not all groups of surgical patients are represented in equal measure when it comes to identifying surgery related PU development. For example, Gao et al. (2018) found that patients undergoing a cardiopulmonary bypass during surgery, had a 6.89-fold increase in PU risk, compared to those without cardiopulmonary bypass (OR =6.89, p<.01)9. However, despite presenting with a 25-30% prevalence of PU, the development of PU in cardiac surgery patients could be more adequately addressed in clinical trials10,11,12 Additionally, a systematic review highlighted that both cardiac and hip surgery patients represent cohorts that are at an increased risk of PU development, which could possibly be due to the age profile of patients undergoing these specific surgeries, combined with the increased duration of time required for these procedures13 This brief glance at the literature shows clearly that cardiac and hip surgery patients require a closer consideration in terms of clinicians thinking about PU prevention.
Some more specific patient factors require clinical attention when patients are to undergo surgery and one of these is pain. A prospective cross-sectional exploratory study by Nilsson (2013) investigated risk factors for postoperative pain and PU associated with supine positioning, in patients undergoing general anaesthesia. A total of 5% (n=4) of patients had reported pain
in their heels, 50% (n=2) of which had developed bilateral grade 1 PU14. Reported pain before the surgical procedure was found to be a significant risk factor for postoperative pain (p=.017 OR 13.1 95% CI 1.4-23.9). This single study places an emphasis on the need for clinicians to assess pain in patients who are undergoing surgery, as this pain may be directly linked to subsequent PU development. This is because pain is an indicator of an early inflammatory response, and if the patient has this already going in to the operating room, they will not be able to tolerate further insults arising from pressure and shear during surgery15
Although the duration of the procedure is a dominant feature linked to PU development related to surgery, it is clear that there are many other factors involved in the development of surgical related PU. Thus, it is imperative that clinicians implement preventive interventions to manage and minimise these risks. Identifying those who need prevention strategies is a critical first step, and this is achieved through risk assessment16. Visual skin assessment and risk assessment scales are the most widely used methods to determine PU risk for those undergoing surgery3,17,18. However, a greater challenge is capturing PU development and managing PU risk during the intraoperative period. Recently, Martins de Oliveira et al., (2022) have examined the predictive ability of subepidermal moisture (SEM) measurement versus traditional risk assessment and visual skin assessment for PU detection among adults undergoing surgery. This non-experimental, comparative, descriptive cohort study showed that the variables which emerged as statistically significant relating to abnormal SEM measurement deltas among these participants were: surgery duration (p=0.038); having orthopaedic surgery (p=0.020); supine surgical position (p=0.003); spinal anaesthetic type (p=0.0001); and Waterlow and Braden mobility subscale day one postoperatively (p=0.0001)17 Crucially, Martins de Oliveira et al., (2022) draw
“Pain is an indicator of an early inflammatory response, and if the patient has this already going in to the operating room, they will not be able to tolerate further insults arising from pressure and shear during surgery.”
attention to the risk assessment of patients using the Braden and Waterlow scales. They point out that the use of the Braden and Waterlow risk assessment scales for surgical patients should be reassessed by placing greater consideration on mobility as a risk factor for surgical patients and focusing on cellular responses to pressure and shear identified through SEM measurements17. In a nutshell, although the use of a risk assessment tool is recommended by international PU prevention guidelines, existing tools are limited in their scope and ability to 100% accurately predict the occurance of a PU in all cases19. However, recent trends, based on technological methods of predicting risk, such as the measurement of SEM, offer some light in the search for a clinically viable and more reliable method of predicting PU risk, particularily in surgical patients.
1. Khor HM, Tan J, Saedon NI, Kamaruzzaman SB, Chin AV, Poi PJ, Tan MP. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr. 2014 NovDec;59(3):536-41. doi: 10.1016/j.archger.2014.07.011. Epub 2014 Jul 21. PMID: 25091603.
2. Walker RM, Gillespie BM, McInnes E, Moore Z, Eskes AM, Patton D, Harbeck EL, White C, Scott IA, Chaboyer W. Prevention and treatment of pressure injuries: A meta-synthesis of Cochrane Reviews. J Tissue Viability. 2020 Nov;29(4):227-243. doi: 10.1016/j.jtv.2020.05.004. Epub 2020 Jun 28. PMID: 32624289.
3. Peixoto CA, Ferreira MBG, Felix MMDS, Pires PDS, Barichello E, Barbosa MH. Risk assessment for perioperative pressure injuries. Rev Lat Am Enfermagem. 2019 Jan 17;27:e3117. doi: 10.1590/1518-8345.2677-3117. PMID: 30698218; PMCID: PMC6336361.
4. EPUAP, N., PPPIA 2019. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline.
5. SCHOONHOVEN, L., DEFLOOR, T. & GRYPDONCK 2002. Incidence of pressure ulcers due to surgery. Journal of Clinical Nursing, 11, 479-487.
6. BULFONE, G., MARZOLI, I., QUATTRIN, R., FABBRO, C. & PALESE, A. 2012. A longitudinal study of the incidence of pressure sores and the associated risks and strategies adopted in Italian operating theatres. J Perioper Pract, 22, 50-6.
7. SCHOONHOVEN, L., GROBBEE, D. E., DONDERS, A. R., ALGRA, A., GRYPDONCK, M. H., BOUSEMA, M. T., SCHRIJVERS, A. J., BUSKENS, E. & PRE, P. S. G. 2006. Prediction of pressure ulcer development in hospitalized patients: a tool for risk assessment. Qual Saf Health Care, 15, 65-70.
8. LUMBLEY, J. L., ALI, S. A. & TCHOKOUANI, L. S. 2014. Retrospective review of predisposing factors for intraoperative pressure ulcer development. J Clin Anesth, 26, 368-74.
9. GAO, L., YANG, L., LI, X., CHEN, J., DU, J., BAI, X. & YANG, X. 2018. The use of a logistic regression model to develop a risk assessment of intraoperatively acquired pressure ulcer. Journal of Clinical Nursing, 27, 2984-2992.
10. CHELLO, C., LUSINI, M., SCHILIRO, D., GRECO, S. M., BARBATO, R. & NENNA, A. 2019. Pressure ulcers in cardiac surgery: Few clinical studies, difficult risk assessment, and profound clinical implications. Int Wound J, 16, 9-12.
11. RAO, A. D., PRESTON, A. M., STRAUSS, R., STAMM, R. & ZALMAN, D. C. 2016.
Risk Factors Associated With Pressure Ulcer Formation in Critically Ill Cardiac Surgery Patients: A Systematic Review. J Wound Ostomy Continence Nurs, 43, 242-7.
12. FEUCHTINGER, J., HALFENS, R. J. G. & DASSEN, T. 2005. Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart & Lung, 34, 375-385.
13. CHEN, H. L., CHEN, X. Y. & WU, J. 2012. The incidence of pressure ulcers in surgical patients of the last 5 years: a systematic review. Wounds, 24, 234-41.
14. Nilsson UG. Intraoperative positioning of patients under general anesthesia and the risk of postoperative pain and pressure ulcers. J Perianesth Nurs. 2013 Jun;28(3):137-43. doi: 10.1016/j.jopan.2012.09.006. PMID: 23711309.
15. Wilson H, Moore Z, Avsar P, Moda Vitoriano Budri A, O’Connor T, Nugent L, Patton D. Exploring the Role of Pain as an Early Indicator for Individuals at Risk of Pressure Ulcer Development: A Systematic Review. Worldviews Evid Based Nurs. 2021 Aug;18(4):299-307. doi: 10.1111/wvn.12528. Epub 2021 Jul 24. PMID: 34302432.
16. H. & MOORE, Z. 2022. Sub-epidermal moisture versus traditional and visual skin assessments to assess pressure ulcer risk in surgery patients. J Wound Care, 31, 254-264.
17. MARTINS DE OLIVEIRA, A. L., O’CONNOR, T., PATTON, D., STRAPP, O’CONNOR, T. 2017. Subepidermal moisture (SEM)
18. WHITEING, N. L. 2009. Skin assessment of patients at risk of pressure ulcers. Nurs Stand, 24, 40-4.
19. Moore ZE, Patton D. Risk assessment tools for the prevention of pressure ulcers. Cochrane Database Syst Rev. 2019 Jan 31;1(1):CD006471. doi: 10.1002/14651858.CD006471. pub4. PMID: 30702158; PMCID: PMC6354222.
20. HETTRICK, H., HILL, C. & HARDIGAN, P. 2017. Early Detection of Pressure Injury Using a Forensic Alternate Light Source. Wounds, 29, 222-228.
“Recent trends, based on technological methods of predicting risk, such as the measurement of SEM, offer some light in the search for a clinically viable and more reliable method of predicting PU risk, particularily in surgical patients.”
This Masterclass Guide is an overview of the Bruin Biometrics Provizio
SEM Scanner. It explores how the device works and considers supporting evidence for its usage.
Pressure injury/ulcer (PI/U) can develop over minutes to hours. The Provizio® SEM Scanner targets a specific stage in the PI/U cascade1 where localized changes in the biocapacitance properties of tissues at risk are identified; these changes reflect the inflammatory response and developing localized oedema and may be reversible1
Currently, clinicians make subjective visual skin assessments (VSAs) to diagnose PI/U. SEM Scanner technology acts as an adjunct to current standard of care such as Visual Skin Assessment and Risk Assessment Tools. Results from the device enables early initiation of preventative measures and treatment.
Compared with visual skin assessment the device has been shown to enable healthcare providers to detect specific anatomical areas that are at increased risk of PI/U 5 days (median) earlier than VSA3.
A vital role of SEM assessment technology is the early identification of increased risk of PI/U in darker skin tones4
■ The device identifies changes in the biocapacitance of tissue which may indicate early pressure damage, before the injury is visible on the surface of the skin
■ It specifically identifies localized oedema (SEM), a biomarker which notifies practitioners of incipient damage, even if the skin and tissue are not exhibiting other signs of PI/U damage, such as erythema - this is early, actionable information1
■ A small, noninvasive, wireless hand-held device and a charging hub (figure 3)
■ It includes a single-use sensor, a patient bar coded wrist band scanner and a display screen
■ The sensor is single-use, and can be quickly and easily replaced between patients (figure 5)
■ The device may be used with dedicated software (Provizio® SEM Scanner Gateway), which can be installed on a WiFi network
■ The device can scan a bar code wrist band worn by a patient, and uploads the data to the Gateway Dashboard, when the device is returned to the charging hub
■ The Barcode Button (figure 1b) allows the patient’s barcoded wrist band to be scanned after which, the SEM data is uploaded when the device is returned to the charging hub, providing the Provizio® SEM Scanner Gateway is installed on a WiFi network
■ The Manual Charting Button is intended for use when bar coded wrist bands are not available or Provizio® SEM Scanner Gateway is not installed
■ The Manual Patient ID Button allows manual entry of the patient ID

■ A training button is also available for training purposes
1 Turn on the device, either by removing the device from the charging hub, or pressing the action button. The splash screen will display
2 Shortly, the replace sensor screen will display. Remove the new sensor from the packaging and place on the sensor head, ensuring you hear a click which indicates it is installed correctly
Press the ‘next screen’ button, and choose the mode of operation (figures 1a and 1b)
4 The display will move to the Body Location Selection Screen, where you may select the sacrum, left or right heel
5 Begin the scan (figure 2, figure 4)
6 Once the scan is complete, press the home button. The display will return to the replace sensor screen if using the single-use sensor
7 Remove the single-use sensor by gently pulling it from the sensor head
Clean and disinfect the scanner, following the instructions in the User Manual (Link to User Guides)
Place the device in the charging hub to initiate data upload
10 Charge the device by placing it into the charging hub (figure 3). A green light confirms the scanner is charging correctly
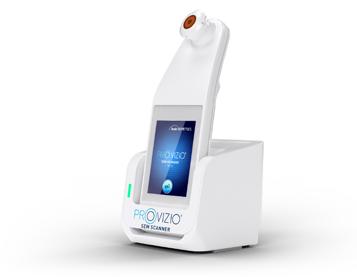
and sensitive on darker
■ Ensure that any surface moisture or matter is removed from the area on the skin being assessed
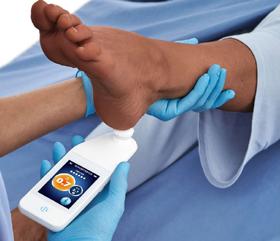

■ Apply the sensor flat against the patient’s skin in the area to be scanned with sufficient pressure until the scan is triggered (figure 2)
■ When a successful reading is taken, the scanner will flash blue and emit a short audio tone
■ Lift the sensor off the skin. The session circles above the body location map will turn white when all measurements are complete
■ Repeat above steps to obtain a complete set of readings for sacrum, left and right heel
■ Δ ≥0.6 may suggest increased risk of PI/U. The value should be considered in conjunction with clinical judgement
■ Press the Body Location Selection Screen Button (figure 6) to return to the screen

PI/U prevention is based on a challenged standard of care (SoC) pathway. An over-reliance on skin and tissue assessments as the trigger to anatomy specific interventions, only once the skin redness has developed, needs to be challenged.
SEM technology as an assessment tool can assess signs of physiological tissue damage in real time. This technology has been one of the most widely evidenced technologies in PI/U prevention1
■ In a multicentre PI/U reduction programme (PURP)2 conducted to determine the impact upon PI/U incidence reduction following implementation of the SEM assessment technology, 72% of 2,232 patients in the acute care cohort received additional interventions as a result of the objective data delivered by the scanning device; clinical decision making was impacted in 69% of cases. In the hospice-care cohort a 47% reduction in PI/U incidence rates was noted. In the community care cohort, reductions in community acquired PI/U across two community care settings were 27% (patients at home) and 100% (community hospital). The authors write: “In acute care sites, implementing the SEM Scanner into routine clinical practice has resulted in an overall 90.5% reduction compared to prior incidence rates.”
■ Considering the efficacy of the SEM assessment technology, Peko and Gefen (2019) reported successful detection of fluid level changes in a tissue area as small as 1mm; these results were reproducible5,6
■ An analysis of data from 15,574 assessment involving 1995 patients found that use of the SEM device resulted in more nurse action to prevent PI/U than skin and tissue assessments (STAs) alone7 The multilevel model revealed strong evidence that SEM Δ prompts were significantly associated with nurse action (p<0.001; adjusted odds ratio: 1.99)
■ After conducting a study on the effects of introducing SEM scanning technology into SOC, Nightingale and Musa (2021) noted the benefits of access to objective and accurate data via the SEM scanning device in decision making by clinicians, in relation to the importance of early intervention in prevention of PI/Us8
■ In a comprehensive cost–benefit analysis Gefen et al (2020) used a probabilistic cost–benefit model in the UK NHS setting9
■ Two alternate acute hospital scenarios of lower (1.6%) and higher (6.3%) PI/U incidence rates were assessed, and it was concluded that implementation of SEM Scanner technology would likely lead to significant financial benefits and downstream cost savings in the order of £15.23 and £80.68 per admission, respectively. Estimated total savings from SEM Scanner utilization for an average UK Trust with 40,802 admissions yearly would range between £0.6 and £3.3 million annually9
■ Using a Markov modelling technique Padula et al.,10 concluded that use of SEM technology in the US setting was associated with cost savings of US $4054 per acute care admission, avoiding the costs associated with PI/U in healthcare facilities, and possibly seeing a return on investment in under a year. The authors note: ”SEM Scanners as part of a prevention protocol are a dominant strategy compared to standard care since it lowers costs and increases Quality-Adjusted Life Years (QALYs).”
■ Portability, wireless connectivity, and ease of use5,9 means that the device can be utilized by individual staff members, benefiting time management and overall costefficiency in a healthcare facility
■ The device is small, wireless, portable and easy to use
■ Peko and Gefen (2020) discuss the improvements in the newest version of the device, noting the ability to perform scans on small areas of the body such as the heels, due to smaller sensor size; beneficial overall reduction in size of the device and the user-friendly interface, and improved wireless connectivity6
“The estimated total financial savings from implementing the SEM Scanner... would range between £0.6m to £3.3m per annum.” Gefen, 2020
“The SEM Scanner technology has proven costeffectiveness, demonstrated in comprehensive published work.” Gefen, 2020
“...wide implementation of the SEM Scanner technology... is well justified from a financial perspective and will lead to cost savings.” Gefen, 2020
“SEM biocapacitance measures can complement visual STAs, facilitate earlier identification of the risk of specific anatomies developing PIs, and inform earlier anatomy specific intervention decisions than visual STAs alone.” Okonkwo, 2020
“Implementing scanning technology into routine clinical practice achieves consistent reductions in pressure injury/ulcer incidence.”
& Musa, 2021
1. Gefen A. The SEM Scanner for early pressure ulcer detection: a 360-degree review of the technology. Wounds International. 2020; 11(4):22-30. https://www.woundsinternational.com/ resources/details/sem-scanner-early-pressure-ulcer-detection-360-degree-review-technology (accessed 2 June 2022)

2. Bryant RA, Moore ZE, Iyer V. Clinical profile of the SEM Scanner - Modernizing pressure injury care pathways using Sub-Epidermal Moisture (SEM) scanning. Expert Rev Med Devices. 2021 Sep;18(9):833-847. doi: 10.1080/17434440.2021.1960505. Epub 2021 Sep 3. PMID: 34338565.
3. Okonkwo H, Bryant R, Milne J, Molyneaux D, Sanders J, Cunningham G, Brangman S, Eardley W, Chan GK, Mayer B, Waldo M, Ju B. A blinded clinical study using a subepidermal moisture biocapacitance measurement device for early detection of pressure injuries. Wound Repair Regen. 2020 May;28(3):364-374. doi: 10.1111/wrr.12790. Epub 2020 Jan 21. PMID: 31965682; PMCID: PMC7217158.
4. Bates-Jensen BM, McCreath HE, Pongquan V. Subepidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones. J Wound Ostomy Continence Nurs 2009;3 6(3): 277–84
5. Peko Cohen L, Gefen A (2019) Phantom testing of the sensitivity and precision of a sub-epidermal moisture scanner. Int Wound J 16(4): 979–88
6. Peko L, Gefen A (2020) Sensitivity and laboratory performances of a second-generation sub-epidermal moisture measurement device. Int Wound J 17(3):
Validated efficacy1 for early assessment of Pressure Injury (PI) risk and damage in peri-operative patients.
Provizio SEM Scanner delivers objective and anatomically-specific assessment of PI risk, empowering you to deploy a targeted prevention strategy that helps minimise PI incidence and reduce overall cost and time to care. This is especially important for peri-operative patients who, due to periods of immobility, can be particularly vulnerable to the effects of pressure and shear.
Findings from the latest publication1 assessing the use of the SEM Scanner on a sample of 231 surgical patients:
• Using the SEM Scanner, 51% of patients presented high SEM readings indicating early development of PI (n=116)
• Using the Braden Scale, only 16% of patients were identified as At Risk (n=37)
”Because SEM measurement is a diagnostic tool that can detect early risk and damage, those undergoing surgery should have the areas of their bodies that have been subject to pressure/shear scanned immediately postoperatively and every day until discharge, or until SEM deltas normalise.” 1
The use of sub-epidermal moisture (SEM) assessments is recognised in the EPUAP - NPIAP - PPPIA International Clinical Practice Guidelines since 2019. 2
* Median
References: 1. Oliveira M, Lúcia A et al. Sub-epidermal moisture versus traditional and visual skin assessments to assess pressure ulcer risk in surgery patients. Journal of wound care vol. 31 Issue 3 (2022), P. 254-264. doi:10.12968/jowc.2022.31.3.254. 2. EPUAP/NPIAP/PPPIA (2019). Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. Section 5, p.78-79.
For more information visit www.arjo.com/Provizio
Patients with chronic and hard-to-heal wounds, such as diabetic foot ulcers and venous leg ulcers, experience significant morbidity such as pain, and loss of function. Initial management includes debriding necrotic tissue, applying dressings that maintain a moist wound environment, treating any concurrent wound infections, and restoring blood flow to the wound site. If these procedures fail to restore the healing process, additional therapies may be considered.
Chronic wounds fail to heal due to a variety of systemic and local factors including high microbial burden and excessive devitalized tissue1.
Patients with chronic and hard-to-heal wounds, such as Diabetic Foot Ulcers (DFUs) and Venous Leg Ulcers (VLUs), experience significant morbidity such as pain, and loss of function. Initial management includes debriding necrotic tissue, applying dressings that maintain a moist wound environment, treating any concurrent wound infections, and restoring blood flow to the wound site. If these procedures fail to restore the healing process, additional therapies may be considered.
Healing becomes compromised once bacteria from the environment invade and biofilm forms2
• Biofilm is an assemblage of surfaceassociated microbes enclosed in a selfproduced matrix.
• Identifying and managing biofilm is a crucial component of successful wound care protocols2.


Skin substitutes can be categorised based on the Davison-Kotler classification system. Briefly they can be categorized as synthetic skin substitutes, acellular dermal substitutes derived from human placental membranes, cellular dermal substitutes, cellular epidermal and dermal substitutes, and animal tissue sources. DFUs, Pressure Ulcers (PUs),
and VLUs can all be treated with skin substitutes.
Wound treatment with PHMB antimicrobial barrier (PCMP)* can safely and effectively manage bacterial bioburden3,4.
PuraPly® Antimicrobial Wound Matrix (PuraPly® AM) consists of a collagen sheet coated with 0.1% Polyhex-Methylenebiguanide hydrochloride (PHMB) intended for the management of wounds. PuraPly® AM is supplied dry in sheet form. The device is packaged in sterile, sealed single pouches.
PuraPly® AM Wound Matrix is a native, crosslinked ECM plus PHMB antimicrobial barrier, intended for the management of acute and chronic wounds including partial- and fullthickness wounds, pressure ulcers, surgical wounds, trauma wounds, and venous and diabetic ulcers.
This study aimed to provide foundational data on the effects of a native cross-linked extra cellular matrix with PHMB antimicrobial barrier (PCMP)* on modulating bioburden and reformation of biofilm to support wound healing.
• 5 subjects >18 years old with an open wound of the lower extremity of various etiologies (DFU, VLU, latrogenic) were included.
Subjects had weekly clinical visits for the 6-week study period incorporating
debridement followed by PCMP application.
• Secondary dressings were applied to maintain a moist wound environment.
• DFUs on weight bearing areas were offloaded and all VLUs were compressed.
• A fluorescence imaging device** captured fluorescence images to assess high levels of bacterial contamination and standard images with measurements5
The mean baseline wound duration was 23 weeks, and mean baseline size was 7.86 cm2; two of the 5 wounds healed by week 4.
The mean percentage area reduction of all wounds was 59.7% by week 4, and 78.1% by week 6. All wounds displayed positive fluorescence on week 1, progressing to negative fluorescence by week 4.
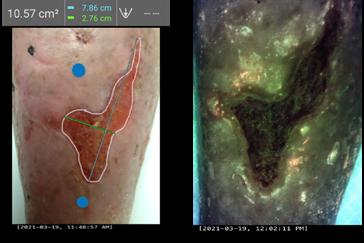
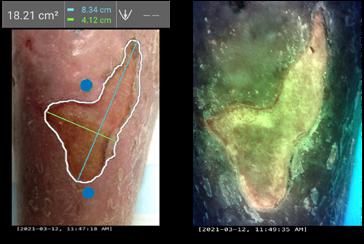
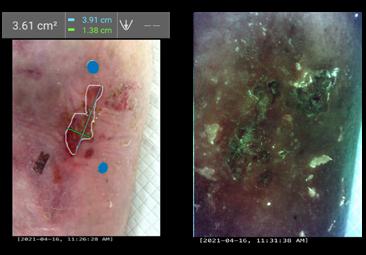
PCMP was able to resist proteolytic degradation and retain a sustained antimicrobial barrier effect.
The results of this pilot study support the hypothesis that
of PCMP into current clinical pathways supports the wound healing environment, through the resolution of bacterial burden and
reformation, to improve patient outcomes.
A larger study group should be considered that reports
such as
outcomes, such as recurrent wounds,
This
in
of
(NPWT),
Soft tissue reconstruction of the diabetic foot is a challenge for clinicians. Primary closure is not an option and healing with secondary intention is not always optimal.
Other options include local flap closure, skin grafts, pedicled flaps and free tissue transfer.
This article provides an overview and comparison of biological and synthetic dermal matrices, and a summary of the advantages of synthetic dermal matrices, supported by two case studies.
There is a diabetic foot ulceration epidemic occurring, with up to 25% of diabetics in the USA going on to develop a foot ulcer in their life. The total cost of diabetes in the USA is $176 billion, with an estimated annual cost for diabetic foot ulceration of $15 billion2
Similarly, in the UK alone there was an increase from 176,000 to 342,000 patients with diabetic foot ulceration3. This is a staggering 95% rise from 2012/ 2013 to 2017/ 18, which equates to a 20% increase in diabetic foot ulcer (DFU) patients each year due to a combination of new ulcerations and non-healing wounds.

Historically, dressings result in macrounorganised wounds, they must be discarded once changed, and require multiple dressing changes, can cause thermal disruption, and need to be altered depending on the wound type.
Dermal matrices aid the production of micro-
organised wounds. These types of devices require only one application and only the sealing membrane must be discarded. During integration of the matrix, the wound is temporised, and fenestration allows for management of wound exudate and optimal wound healing.
When comparing biological and synthetic matrices, synthetic matrices have clear advantages.
• Biological matrices consist of collagen, which can act as an active food source for bacteria.
• They have a low reliability against infection.
• They are expensive.
• There are ethical and religious considerations.
Synthetic matrices are produced from inert materials.
They are more cost-effective to manufacture.
There are few barriers to their use.
• They are robust in the presence of infections.
NovoSorb® BTM is a fully synthetic device with all components made of polyurethane. It has a sealing membrane that physiologically closes the wound, providing a physical barrier from external contamination. It has fenestrations which allows the drainage of excess fluid that could interfere with integration or become a nidus for infection.
NovoSorb® BTM has an open cell porous matrix. Neovascularisation occurs in this matrix through the interconnected chambers, allowing infiltration with cells related to wound repair.
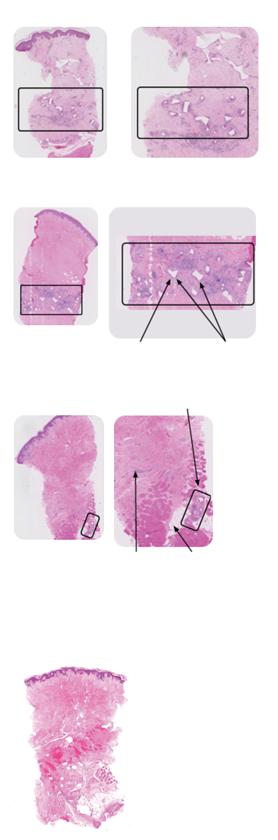
Figure 1 shows a human punch biopsy sample of integrated NovoSorb® BTM. Over time, degradation occurs, and the polymer gradually disappears. By 18 months no residual polymer was seen5.
Some of the benefits of temporising wounds include the prevention of moisture loss, providing a barrier to infection, and physiologically closing the wound until sealing membrane removal and definitive closure performed.
The NovoSorb® BTM matrix has a low density and is approximately 94.2% porous. It has interconnected pores and has multiple interconnections per ‘chamber’. NovoSorb® BTM prevents long collagen links forming, allows free movement of key cells and provides scaffolding for the extracellular matrix (ECM).
The 2mm-thick open cell matrix converts a large complex wound into many tiny ones that the body can heal in an organized manner. Made from a polyurethane polymer, it does not contain any animal collagen, so is not a food source for bacteria, and can often be retained in
Surgical Approach to Healing the Diabetic Foot, Using a Synthetic Dermal Matrix“Some of the benefits of temporising wounds include the prevention of moisture loss, providing a barrier to infection, and physiologically closing the wound until sealing membrane removal and definitive closure performed.”
place if an infection occurs.
NovoSorb® BTM should be applied to a fully debrided wound, trimmed to shape and secured with staples or sutures. Haemoserous fluid from the wound migrates into the matrix, delivering white blood cells, including macrophages and neutrophils, which ‘jump start’ the immune response and stimulate new blood vessel formation. Fibroblasts deposit collagen, building the extracellular matrix which will provide strength and structure for the new tissue.
When the matrix has fully integrated, the sealing membrane can be removed to expose the robust neodermis, which is ready for definitive closure. NovoSorb® BTM progressively biodegrades and is absorbed over time. This material supports the reconstruction of a diverse range of complex wounds, even over exposed bones and tendons, to improve cosmetic and functional outcomes when compared with skin grafting alone.
In this case, NovoSorb® BTM was applied over extensor tendons which were extensively exposed after a traumatic degloving injury6
Figure 2 shows the wound post initial debridement.
Figure 3 shows progress at nine weeks post BTM application. At this stage, the sealing membrane has been delaminated to reveal a robust, well vascularised neodermis ready for grafting.
An ultrasound showed that there was no feature of peritendon inflammation, and that the tendon can be seen to move through the deep and superficial tissues without impediment. The fibrillary structure of the tendon is intact and normal6.
The results nine months after BTM are very satisfactory. The contour allows this patient to wear normal footwear and there is no fixed extension of the joints. Additionally, despite the lack of paratenon prior to reconstruction, the tendons functionally glide under the repair, allowing good toe movement.
In this next case, a 73-year-old Caucasian lady with comorbidities, including non-insulindependent diabetes mellitus, hypertension, and anaemia), developed a wound on her foot from minor trauma. Due to her diabetes and comorbidities, the wound failed to heal and developed into an infected diabetic foot ulcer. Serial debridement was performed, and she was left with a large defect on the dorsum of her foot. NovoSorb® BTM was applied, dressed, and topical negative pressure wound therapy (NPWT) was applied. The patient was discharged after 1 week to a care facility7 (Figure 4).
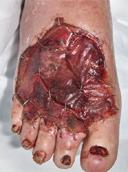
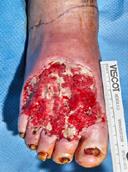
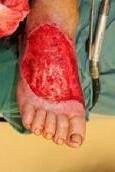
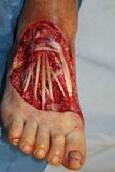 Surgical Approach to Healing the Diabetic Foot, Using a Synthetic Dermal Matrix
One week after NovoSorb® BTM application
Surgical Approach to Healing the Diabetic Foot, Using a Synthetic Dermal Matrix
One week after NovoSorb® BTM application
1. Lazzarini PA, Pacella RE, Armstrong DG, van Netten JJ. Diabetes-related lower-extrem ity complications are a leading cause of the global burden of disability. Diabet Med. 2018 May 23. doi: 10.1111/dme.13680. Epub ahead of print. PMID: 29791033.
2. Diabetic Foot: Facts and Figures [Internet]. https://diabeticfootonline.com; [cited 20 May 2022]. Available from: https://tinyurl.com/9bx55hxp
3. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open. 2020 Dec 22;10(12):e045253. doi: 10.1136/bmjopen-2020-045253. PMID: 33371051; PMCID: PMC7757484.
4. Greenwood JE, Dearman BL. Comparison of a sealed, polymer foam biodegradable tem porising matrix against Integra(R) dermal regeneration template in a porcine wound model. J Burn Care Res. 2012; 33:163-73. doi: 10.1097/BCR.0b013e318233fac1. PMID: 22002205
5. Wagstaff MJD, Schmitt B, Coghlan P, Finkemeyer JP, Caplash Y, Greenwood JE. A biodegradable polyurethane dermal matrix in reconstruction of free flap donor sites: A pilot study. Eplasty. 2015; 15:102–18. https://pubmed.ncbi.nlm.nih.gov/25987938/. PMID: 25987938 PMCID: PMC4412164
6. Damkat-Thomas L, Greenwood JE, Wagstaff MJD. A Synthetic Biodegradable Temporis ing Matrix in Degloving Lower Extremity Trauma Reconstruction: A Case Report. Plast Reconstr Surg Glob Open. 2019 Apr 2;7(4):e2110. doi: 10.1097/GOX.0000000000002110. PMID: 31321161; PMCID: PMC6554176.
7. PolyNovo MK-019 V2.0. Case Report - Infected Diabetic Foot Ulcer. On file.
Problems with the negative pressure wound therapy were encountered. The patient was readmitted with clinical evidence of infection, that was systemically treated. The NovoSorb® BTM was able to be retained despite the infection. Removal of the sealing membrane was performed at 4.5 weeks, a split-thickness skin graft applied, and the patient went on to heal uneventfully.
Globally, millions of diabetic patients with DFU face the imminent prospect of lower limb amputation or death. Synthetic matrices offer an alternate option for reconstruction. Composed of inert materials, robust and cheap to produce, they offer significant advantages in this complex reconstructive challenge.
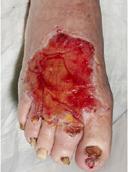
Is
■ NovoSorb® BTM (biodegradable temporising matrix), is a fully synthetic, biodegradable, biocompatible device used for the reconstruction of full or deep partial thickness wounds. It is intended to temporise dermal injuries, where the dermis has been decimated or lost, and to facilitate dermal repair by providing temporary wound closure and a scaffold for the generation of a neodermis

■ NovoSorb® BTM has a bilayer polyurethane design made of a sealing membrane and a 2mm matrix below. The sealing membrane physiologically closes the wound, provides a barrier to bacteria and limits moisture loss1,2

■ The fenestrated membrane allows for free drainage of excess fluid
The 2mm open cell matrix allows cellular infiltration and provides a model for reconstruction of the dermis1

The matrix is made from a polyurethane polymer, so does not feed bacteria. The benefit of this is that it can often be retained if infection is present3
non-
layer
moisture loss, while also
as a barrier to
matrix allows for
serves as a matrix to aid in the
of the
(dermis)


BTM is applied to a wound once the unhealthy or dead tis sue has been surgically removed. BTM temporarily closes the wound, which limits moisture loss2,5
Cellular migration into the matrix results in new blood vessel formation and collagen production throughout the matrix. This normally takes 2 to 3 weeks5
Once a clinician determines the tissue is fully integrated throughout the BTM, the sealing membrane can be removed, leaving a new vascularized dermal layer5
The method of closure will be chosen by the clinician depending on the wound (split-skin graft, moist wound healing dressings, etc). The matrix will retain its structure as it slowly deteriorates over time until fully absorbed at approximately 18 months5,6
NovoSorb® BTM has been the subject of numerous studies and trials, and is well evidenced for efficacy, resistance to infection and cost effectiveness.
■ In a study to explore the potential of NovoSorb® BTM in full-thickness burns patients, Greenwood et al. treated five participants between the ages of 18 and 70, with the device
■ The participants had between 20 and 50% Total Body Surface Area (TBSA) full-thickness burns
■ The BTM was secured using staples and dressed with dressing changes twice a week, with treatment culminating in a skin graft
■ All participants survived and recovered. The scarring in these participants was assessed after a period of six months from time of injury, with excellent results indicated by the scar assessment scales used3
■ Since this early study, there have been many reports by other authors on the clinical effectiveness of NovoSorb® BTM across a range of clinical indications7,8,9
■ The synthetic nature of this device gives it an obvious and significant advantage over biologicallyderived alternatives; the product does not feed bacteria because of this property and it does not have the same risk of immunorejection
■ Sreedharan et al. writes on the successful use of this product in the treatment of large wounds of high risk of infection, involving exposed tendons
■ “Over this (21 day) period, the BTM became gradually more hyperaemic and vascularised. Twenty-four days after implantation the BTM’s surface sealing layer was ‘delaminated’, revealing a vascularised matrix. The right leg anterior compartment tendons were no longer exposed. The wounds were then reconstructed...”8
■ Again, this device is synthetic and does not have a biological origin like most of the products which would be the natural choices for use in the treatment of similar wounds and reconstruction
■ The costs involved in producing the biologically-derived alternatives can be significant, and NovoSorb® BTM is therefore typically available at lower prices, or at least equivalent to cost effective alternatives10
“In each case, our experience of its application, evaluation of integration and split skin graft application, along with the management of complications such as sub-seal infection and haematoma, has steadily increased our confidence in its use.”
“We find the material safe, easy to use, monitor and delaminate. Graft loss over the BTM is extremely unusual.”
“In contrast to other dermal matrices where infection often leads to failure of the material, early infection was able to be successfully treated and the BTM salvaged with a combination of topical wound care and antibiotics.”