Hard-hit Peru’s costly bet on cheap COVID-19 antibody tests
BOGOTA, Colombia (AP) — In the early days of the coronavirus pandemic, the harried health officials of Peru faced a quandary. They knew molecular tests for COVID-19 were the best option to detect the virus – yet they didn’t have the labs, the supplies, or the technicians to make them work.
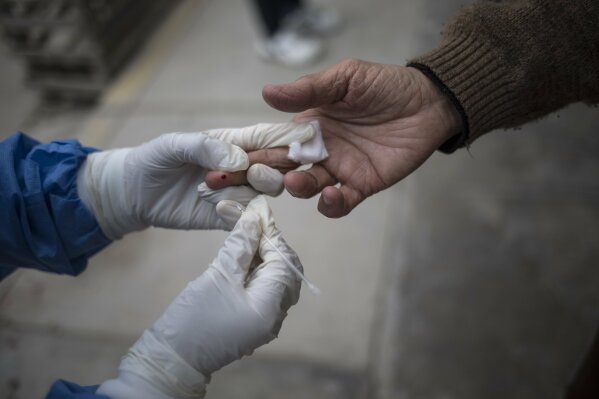
But there was a cheaper alternative -- antibody tests, mostly from China, that were flooding the market at a fraction of the price and could deliver a positive or negative result within minutes of a simple fingerstick.
In March, President Martin Vizcarra took the airwaves to announce he’d signed off on a massive purchase of 1.6 million tests – almost all of them for antibodies.
Now, interviews with experts, public purchase orders, import records, government resolutions, patients, and COVID-19 health reports show that the country’s bet on rapid antibody tests went dangerously off course.
Unlike almost every other nation, Peru is relying heavily on rapid antibody blood tests to diagnose active cases – a purpose for which they are not designed. The tests cannot detect early COVID-19 infections, making it hard to quickly identify and isolate the sick. Epidemiologists interviewed by The Associated Press say their misuse is producing a sizable number of false positives and negatives, helping fuel one of the world’s worst COVID-19 outbreaks.
What’s more, a number of the antibody tests purchased for use in Peru have since been rejected by the United States after independent analysis found they did not meet standards for accurately detecting COVID-19.
Today the South American nation has the highest per capita COVID-19 mortality rate of any country across the globe, according to John Hopkins University – and physicians there believe the country’s faulty testing approach is one reason why.
“This was a multi-systemic failure,” said Dr. Víctor Zamora, Peru’s former minister of health. “We should have stopped the rapid tests by now.”
___
As COVID-19 cases popped up across the globe, low- and middle-income nations found themselves in a dilemma.
The World Health Organization was calling on authorities to ramp up testing to prevent the virus from spreading out of control. One particular test – a polymerase chain reaction exam – was deemed the best option. Using a specimen collected from deep in the nose, the test is developed on specialized machines that can detect the genetic material of the virus within days of infection.
If COVID-19 cases are caught early, the sick can be isolated, their contacts traced, and the chain of contagion severed.
Within weeks of the initial outbreak in China, genome sequences for the virus were made available and specialists in Asia and Europe got to work creating their own tests. But in parts of the world like Africa and Latin America, there was no such option. They would have to wait for the tests to become available – and when they did, the incredible demand meant most weren’t able to secure the number they required.
“The collapse of global cooperation and a failure of international solidarity have shoved Africa out of the diagnostics market,” Dr. John Nkengasong, director of the Africa CDC, wrote in Nature magazine in April as the hunt was underway.
Nations that got an early jump start in preparing or had a relatively robust health care system already in place fared best. Two weeks after Colombia identified its first case, the country had 22 private and public laboratories signed up to do PCR testing. Peru, by contrast, relied on just one laboratory capable of 200 tests a day.
For years, Peru has invested a smaller part of its GDP on public health than others in the region. As COVID-19 approached, glaring deficiencies in Peru became evident. There were just 100 ICU beds available for COVID-19 patients, said Dr. Víctor Zamora, who was appointed to lead Peru’s Ministry of Health in March. Corruption scandals had left numerous hospital construction projects on pause. Peru also faced a significant shortage of doctors, forcing the state to embark on a massive hiring campaign.
Even now, months later, Peru’s needs are vastly under met. To date, the country has less than 2,000 ICU beds, compared to over 6,000 in the state of Florida, which has 10 million fewer inhabitants, according to official data.
High levels of poverty and people who depend on daily wages from informal work complicated the government’s efforts to impose a strict quarantine, further challenging Peru’s ability to respond effectively to the virus.
When Zamora arrived, he said the government had already decided molecular tests weren’t a viable option. The nation didn’t have the infrastructure needed to run the tests but also acted too slowly in trying to obtain what little was available on the market.
“Peru didn’t buy in time,” he said. “Everyone in Latin America bought before us – even Cuba.”
Antibody tests — which detect proteins created by the immune system in response to a virus — had numerous drawbacks. They had not been widely tested and their accuracy was in question. If taken too early, most people with the virus test negative. That could lead those infected to think they do not have COVID-19. False positives can be equally perilous, leading people to incorrectly believe they are immune.
Antibody tests didn’t require high-skill training or even a lab; municipal workers with no medical education could be taught how to administer then.
“For the time we were in, it was the right decision,” Zamora said. “We didn’t know what we know about the virus today.”
___
Ernesto Canayo, a 44-year-old father and city cleaner in Peru’s capital, initially brushed off the fever and headache that wouldn’t go away.
A member of the indigenous Shipibo-Conibo community, he lived in a shack on a hill about 10 blocks from the presidential palace and parliament. The one-room home he shared with his fiancée and 2-year-old son is held together by pieces of wood and plastic tarps.
In early April, at his urging, his family left to stay with relatives outside Lima, believing they’d be better able to avoid the virus. The symptoms started not long after. Over the phone, he told his sister he felt pain in his chest. He went to work, where he was given a rapid test.
“Nothing came up,” he told her.
Worried about losing his job, he continued working, traveling on public transportation, and even joining in members of his community in draining out their shared toilets. The Cantagallo neighborhood – rooster’s crow, in English – does not have regular water or electricity. Since 2013, officials have pledged improvements. Two city mayors who promised housing have been implicated in Latin America’s largest graft probe.
Weeks later, Canayo was still feeling bad and getting worse. Ministry of Health officials arrived at Cantagllo in early May, armed with 120 rapid tests.
Half of those tested came back positive – but not Canayo.
“How can they say I don’t have it?” he asked his sister later. “I feel all the symptoms.”
Throughout Cantagallo, the test caused confusion and alarm. People who seemingly had no symptoms were coming back positive, while others convinced they had COVID-19 tested negative. Those who were positive were told to quarantine, while those who were negative were told they did not have the virus.
“The tests lied,” said Harry Pinedo, an artist and teacher in Cantagallo who said he had numerous symptoms but tested negative. “They deceived us.”
Evelyn Reyes, Canayo’s fiancée, said he told her maybe he was just feeling the effects of the weather. A cold breeze snuck into their unprotected shack at night. He’d barely ever gotten sick; two negative COVID-19 tests cast doubt.
“He told me, ‘I’m fine,’” she said. “‘I’m going to recover from this.’”
___
Antibody tests are designed to be used as serological surveys to provide public health authorities an indication of how widespread the virus is in a community. Some physicians believe they can also be a useful tool if a patient with severe COVID-19 has repeatedly tested negative on a molecular test but has the symptoms. In that case, the virus may no longer be in their airways, but antibodies could be detected.
In Peru, by contrast, the test is frequently being used to make a diagnosis.
“We are not using them in the appropriate way,” said Dr. Rubén Espinoza, former director of Peru’s medical regulation agency. “The results of rapid tests are mentioned as if they were diagnostic ones – which only confuses people.”
Peru’s central government purchased nearly six million antibody tests of four different brands in the initial months of the pandemic, according to public records. At least one, imported from China made by Core Technology Co., does not have approval by China’s medical regulatory agency. It is unclear whether the company sought Chinese regulatory approval; not having it is not necessarily an indicator of quality. The tests can be sold within the European Union because there is no regulatory body to evaluate them before they are placed on the market.
Another, by South Korean company SD Biosensor, has been subject to scrutiny in the U.S.
The group’s Standard Q antibody test was barred for use by the U.S. Food and Drug Administration. The agency does not detail why tests are pulled from distribution; it can be for failing to provide sufficient data or if found not to meet the agency’s standards, among other reasons. An independent analysis of the test sponsored by the National Cancer Institute found that it had only a 76.7% total chance of correctly identifying a positive case.
Peru’s government contends the Standard Q test it purchased is different, because it uses one test strip instead of two, though an FDA spokeswoman said that both are “very similar.”
The state of Rhode Island purchased 20,000 SD Biosensor antibody tests in April but ended up returning them and getting a refund after the FDA barred their use.
The SD Biosensor test – some 5 million of which have been imported to Peru, according to import records compiled by one of the nation’s leading diagnostic companies and shared with The Associated Press – is still being used there. Authorities used it both in the Cantagallo indigenous community and at least one food market in August.
SD Biosensor and Core Technology did not respond to repeated requests for comment.
Dr. Víctor Suárez, subdirector of Peru’s National Institute of Health, said qualitative studies were performed on all the central government’s purchased tests, and that any that did not meet the country’s requirements were sent back. But those tests account for only about half of all rapid antibody tests that have been conducted in Peru. Millions more have been imported from dozens of other brands, many of which are FDA barred.
Suárez said the government’s regulatory agency has done sample surveys of non-state purchases and issued alerts to remove many as a result. Public alerts have been issued for about a dozen tests but not all that have been called into question.
He said that despite the questions raised about individual brands, overall they have helped Peru in identifying more cases than it would have otherwise.
“We’ve done the best we could to identify the most cases possible,” he said. “Whether it’s through molecular tests or rapid ones.”
Ten of the roughly 80 different brands of antibody tests imported are prohibited in the U.S., the records show. A number were bought from relatively small, new companies that were awarded hefty contracts. One company that contracted with the Peruvian government until recently advertised itself as a cosmetics brand.
Emergency declaration regulations have made it easier to obtain contracts and fewer safeguards to protect against corruption are in place, several physicians and people who work in the pharmaceutical industry said.
“They brought in tests from just any company,” said Victor Chu, head of Diagnostica Peruana, one of the older pharmaceutical companies in the country. “Anyone can buy the brand they want, the quantity they want, with hardly any control.”
___
The FDA – which countries like Peru routinely turn to for guidance – was initially lax on what antibody tests were allowed on the market.
The agency had been criticized for acting too slowly in making molecular tests available. When antibody exams entered the picture, it opted to move fast. U.S. regulators allowed the blood tests to hit the market without first providing proof that they worked. They were only required to notify the FDA of their plans and provide disclaimers.
By May, the FDA announced it was rescinding that policy as part of a crackdown on the quality of products introduced on an emergency basis.
“We, unfortunately, see unscrupulous actors marketing fraudulent kits and using the pandemic as an opportunity to take advantage of Americans,” FDA Deputy Commissioner Anan Shah said.
But Americans weren’t the only ones who were sold on faulty products.
In Spain, England, India, and elsewhere, regulators have turned back rapid antibody test purchased and found to yield too many false positives or negatives, or both.
Less developed countries without a strong system to validate the veracity of tests were slower to remove them, and some are still authorized for use today.
“Clearly this is a mistake that the FDA, that the U.S. made, that is having a significant negative ripple effect in other countries,” said Peter Pitts, a former FDA associate commissioner. “Every country that relied on the FDA needs to admit that they made a mistake – that they were inappropriately convinced that these tests were accurate.”
He added: “Bad data only helps the virus spread faster. Bad data costs lives.”
Peru isn’t the only country that has imported potentially faulty tests. In Ecuador, Chile, Mexico, and elsewhere in Latin America, lists of authorized antibody tests still include some of those on the FDA’s banned list. Their governments did not respond to requests for comment.
The specific brand of antibody test being used is critical, advised the Infectious Diseases Society of America, a professional organization of medical specialists. Lateral flow assays – like the blood prick test most commonly used in Peru – are notably inconsistent. Even for surveillance purposes, the society warned it is vital to have tests with a 99.5% or higher specificity, the ability to correctly identify negative cases.
Independent studies sponsored by the National Cancer Institute found some of the tests now removed from the U.S. market but still in use in other countries like Peru had low rates of correctly identifying a positive or negative case.
“Many people who are sick think they are negative, and they’re not,” Chu said. “The government puts labels on food, warning about the quantity of fat. Why not do the same for rapid tests, when this is a matter of life or death?”
The society also urged medical professionals to use antibody tests three or four weeks after the onset of symptoms to avoid incorrect results. In Peru, by contrast, the tests are often being used within days or just a week after symptoms.
“I would not make the conclusion they are all bad,” said Dr. Angela Caliendo, a board member of the society. “I would make the conclusion that some perform strikingly better than others. And if you are going to use – look very closely at the performance statistics.”
___
In Cantagallo, the nearly 250 families who live packed in small huts grew even sicker after the state’s first round of COVID-19 testing.
When government workers returned 10 days later, they detected far more cases; of 656 people given an antibody exam, 476 came back positive, or nearly 73%. People who had tested negative like Canayo hadn’t properly isolated themselves. A long-standing skepticism of hospitals meant the sickest refused to seek help. A people of close-knit ties, they were also reluctant to leave those who tested positive alone.
Instead, families kept their sick nearby, trying to help them recover with natural remedies like inhaling the vapors of boiled eucalyptus leaves.
“Everyone was sick,” said Pineda, the artist. “Everyone had COVID.”
Images from a day of mass testing in May show technicians used a test by Chinese company Coretests. Relatively little information can be gleaned from its website or in published independent studies. The test is not on the FDA’s barred list, but is not approved either. It is not approved for use by China’s own medical regulatory agency.
Canayo, meanwhile, continued to grow worse. He had all the classic COVID-19 symptoms, including loss of his sense of taste. His fiancée said he took Tylenol but likely needed oxygen, which the community did not have.
The day before Mother’s Day, Rossi Canayo, 40, called to check up on her brother. Though she couldn’t see him, she could tell from the sound of his voice that he was ill and agitated.
Neighbors came to check on him but his fiancée and sister were nowhere near, trapped in towns far away amidst the nation’s strict lockdown.
“He was alone,” she said.
___
Across Peru, many families were grappling with similarly confusing results.
Janina Huallpa, a phlebotomist for a children’s cancer clinic, came down with a fever in May. She’d been out working in the early months of the pandemic -- going to bus stops, supermarkets, and other crowded areas searching for blood donors who could help save lives.
A rapid test administered at work came back negative. Dubious, she went to a public hospital, where another antibody test also delivered a negative result.
“They told me maybe it was an infection,” she said.
Over the next three weeks, she got progressively worse, struggling to walk and breathe. Again, she went to the hospital and again she tested negative and was sent home. Doctors told her she didn’t have the virus.
Worried and anxious, the mother of two went back to the hospital days later when she didn’t improve. This time, doctors did a chest X-ray and found pneumonia. A fourth rapid test came back negative, but doctors nonetheless decided to send her to a larger hospital in Lima, where she got a molecular test.
The test came back positive and she spent 10 days on a ventilator.
When she woke up, doctors told her that eight other people who entered the ICU on the same day she did had all died of COVID-19.
“If one of those tests had been positive, I would have gotten adequate treatment,” Huallpa said. “How many people are dying because of this?”
___
To date, Peru has diagnosed over 800,000 people with COVID-19; 77% of those cases have been diagnosed through rapid antibody tests. Overall, it has conducted over 8 million blood tests – and just 780,000 nasal swab molecular ones.
In the world’s hardest-hit region, it is the country with the third highest number of cases, nearly on par with Colombia, which has almost 20 million more residents.
More than 32,000 people have died, but health officials admit 23,585 more with COVID-19 symptoms may have perished before getting a positive test. Even by the lowest statistic, Peru has about as many deaths as France, which has a population twice as large.
Some physicians are reluctant to chastise the country’s heavy reliance on antibody testing. They contend that, when used correctly, the tests can be a helpful tool, particularly for nations like Peru that do not have wide molecular testing capability. In addition, several said doctors should be making a diagnosis based on symptoms, not a test – and that multiple factors including years of underinvestment in health care are ultimately to blame.
The nation has made advances in expanding molecular testing; there are now 46 public and private labs capable of processing the exams. In addition, Peru recently began using a molecular test created by doctors within the country that can deliver results within two hours.
“Rapid tests have their place and their moment for use,” said Pilar Mazzetti, Peru’s minister of health. “No test can detect the virus 100% of the time.”
Even though Peru can now do 12,000 molecular tests a day – still far fewer than many other countries in Latin America – it continues to diagnose primarily through antibody tests. Physicians said that widespread misperceptions about the significance of a positive or negative result on an antibody test are leading to grave mistakes.
“The use of rapid tests hasn’t changed, which is unacceptable to me,” said Dr. Ernesto Gozzer, a professor at the Cayetano Heredia University in Peru.
At a wide range of jobs– from photo shoots to food markets – employers require a negative antibody test in order to return to work. The tests – which can cost $30 each – have become popular. Yet a positive result isn’t necessarily a sign of an active infection. Rather, it can indicate that someone had previously had the virus.
Likewise, a negative result offers no assurance that someone doesn’t have the virus.
Molecular tests run at about $110 each in Lima, or nearly half the monthly minimum wage, making them out of reach for many Peruvians who can’t pay out of pocket and might only be offered a serological test at a local clinic.
Construction supervisor Marco Mayo saw firsthand the haphazard results the tests can generate. He used two different tests sold under two different brands -- one on his left index finger and the other on his middle finger. One finger came back positive, the other negative.
He was not allowed to return to work while the results remained unclear.
“I lost a month of my life because of this,” he said.
Other bad results have been far more consequential.
Katy Retamozo, president of the Peruvian Association of ICU nurses, said she has seen patients that tested negative on rapid antibody exams return days later with advanced COVID-19 pneumonia. The delay in properly identifying cases, she believes, could be a factor in the nation’s especially high level of mortality.
“That made them delay getting to the hospital,” she said.
___
At 5 a.m. on Mother’s Day, Rossi Canayo’s cell phone rang. On the other end, a voice relayed the news: Her brother was dead.
Word of Ernesto’s death struck fear in Cantagallo. Suddenly, the virus many had dismissed as a distant threat was in their midst. And if an otherwise healthy adult who tested negative could die a week later, all of them were vulnerable.
For his sister, his death has stirred up questions and anguish. She wonders what would have happened if a test had quickly diagnosed his illness. And she also questions why the city he worked for used an antibody test on a worker who was out in the streets, with symptoms.
“They abandoned him,” she said.
__
Associated Press researcher Chen Si in Shanghai, China contributed to this report.
Contact AP’s global investigative team at [email protected].