H. pylori affects millions of people worldwide, and while there are a number of testing options, stool antigen testing offers a better choice due to its accuracy and cost effectiveness.
By Jodie Y. Lee, MS, MBA, and David M. Lyerly, PhD
Helicobacter pylori (H. pylori) infects billions of people of all ages, nationalities, and socioeconomic statuses around the world. Those who become infected may carry the organism throughout their entire lives. Some people become ill from the infection, but the majority do not. Many experts believe that infection with the organism occurs primarily in childhood, although transmission among adults also occurs. The high positivity rates reported in prevalence studies are based on data obtained through serology testing, meaning that large numbers of people globally have been exposed to H. pylori. The numbers are staggering. In North America, 30% to 40% of the population has been exposed; outside North America, the rates approach 70% or more.
H. pylori can be passed from person to person, especially among family members, through saliva, fecal contamination, and poor hygiene. Proper hygiene, careful preparation of food, and access to clean water help to slow transmission. In developing countries, factors such as untreated water, crowded conditions, and poor sanitation all contribute to higher prevalence rates.
Portrait of an Unusual Pathogen
H. pylori, a curved and helical human pathogen, has physiologically adapted to live in the harsh acidic environment of the stomach. Morphologically, H. pylori is gram-negative and is highly motile with one or several unipolar flagella. The organism grows slowly but can be isolated on blood agar or grown in broth cultures. H. pylori is a microaerophile and tolerates oxygen, but only at low amounts, which makes culturing the organism from biopsy specimens challenging. Isolates and biopsy specimens can be subsequently screened for urease, oxidase, and catalase, along with other biochemical tests for identification.
The urease of H. pylori, which converts urea in gastric juices to ammonia, is very potent, and enables the organism to survive in the gastric mucosa. When grown in culture, urease may account for up to 5% of the total protein produced, indicating that this enzyme is a major protein of H. pylori. The urease neutralizes stomach acid as it reaches the exterior of the bacterial cell, preventing damage to the inner cellular membrane, protecting the organism.
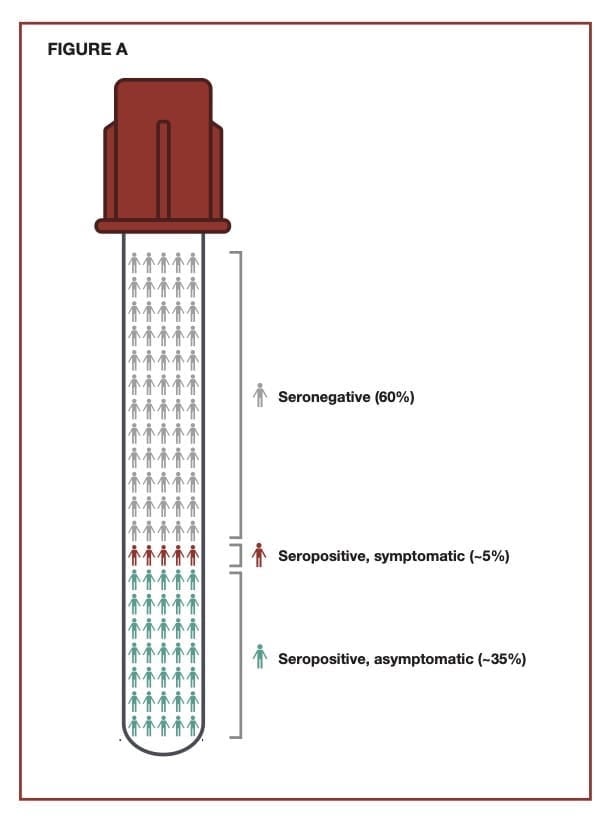
Exhibiting a Wide Range of Symptoms
The major symptoms associated with an H. pylori infection typically include a combination of diarrhea, peptic ulcers, abdominal pain, and black tarry stools. Additionally, there can be minor symptoms such as belching, bloating, nausea, and abdominal discomfort. The severity of any of these symptoms can vary and will overlap with the general diagnosis of gastritis, typically defined as an inflammation in the lining of the stomach. About 10% of persons infected with H. pylori will develop symptoms, and a low percentage will develop gastric cancer caused in large part by ongoing inflammation associated with the chronic infection in the stomach. Most infected individuals will be asymptomatic.
H. pylori is a significant cause of peptic ulcer disease, which is recognized globally as a health problem. The organism weakens the stomach lining, allowing gastric secretions along with the organism to trigger gastric and duodenal ulcers collectively referred to as peptic ulcers. According to the Centers for Disease Control, approximately 25 million people in the U.S. suffer from peptic ulcer disease at some point in their lifetime. Each year in the U.S., there are 500,000 to 850,000 new cases of peptic ulcer disease and more than 1 million ulcer-related hospitalizations. H. pylori causes about 90% of these hospitalizations. Nonsteroidal anti-inflammatory drugs and medications that erode the stomach lining cause the other 10%.
Setting a Testing Profile
According to recent guidelines from the American College of Gastroenterology (ACG), the Houston Consensus Conference on H. pylori, the World Gastroenterology Organisation Global Guidelines, and the American Journal of Managed Care, all patients who test positive for H. pylori should be offered treatment.1-4 This includes those with active peptic ulcer disease, a past history of peptic ulcer disease if symptomatic, low-grade gastric mucosa-associated lymphoid tissue lymphoma, and a history of gastric cancer. More recently, the list has been extended to include persons with reflux/dyspepsia, and those on aspirin and non-steroidal anti-inflammatory drugs as well as persons with a family history of gastric cancer, first-generation immigrants from high prevalence areas, and those of Latino and African American backgrounds.
The guidelines strongly support a “test-treat-test” approach that includes the treatment of persons who test positive, followed by additional testing after antibiotics to confirm eradication of H. pylori. The need to confirm the eradication of the organism is key to this strategy. The failure to eradicate H. pylori most often results from antibiotic resistance of the organism, which is common, although nonadherence by the patient can also be a factor.1 The resistance seen over the past several decades continues to increase and includes resistance to antibiotics such as clarithromycin, levofloxacin, and amoxicillin that are often used as part of the initial regimen.
Weighing Diagnostic Testing Types
There are invasive and noninvasive diagnostic laboratory-based test procedures for H. pylori and its disease. Invasive tests use endoscopy and gastric biopsy for the collection of specimens for laboratory testing using histology staining, culture, and/or urease to confirm the presence of H. pylori.5,6 Histology testing is roughly 60% to 85% sensitive, and culture is less sensitive than histology. Urease testing provides sensitivity values typically >80%, probably closer to 90%, requiring about 105 organisms in the sample for a positive reaction. The test is fairly specific, but other urease-producing bacteria may occasionally cause positive reactions.
Noninvasive tests include the urea breath test, serology tests, and stool antigen testing. Noninvasive tests are becoming more routine because (i) they do not require the collection of biopsy specimens and (ii) the performance of the breath test and stool antigen testing is comparable to results obtained with invasive tests. PCR detection of H. pylori also has been used with various specimens including gastric biopsy, gastric juice, saliva, dental plaque, and stool samples. PCR testing is not used as routinely as the other noninvasive tests, in part because there are no FDA-cleared PCR tests currently available and because PCR testing is more expensive. In addition, DNA degradation may play a role in PCR performance for H. pylori in fecal specimens. At this stage, molecular assays have been utilized more as an epidemiological tool for the detection of antibiotic resistance patterns.
Costs of the tests vary from country to country, especially outside the U.S. and in less developed countries, which factors into the type of tests most commonly used. The urease test is relatively inexpensive and is used frequently in low-income countries, but the costs of biopsy collection can be prohibitive. The noninvasive urea breath test also is used less often in low-income countries because of the higher costs associated with the collection and shipment of specimens, along with specialized equipment needed to measure and dispose of radioisotope-labeled CO2 released by the action of urease.
Serology immunoassays have been used for many years primarily because of availability and low costs. Serology testing is easier on the patient because it is not affected by proton pump inhibitors or antibiotics, and patients can continue these medications during testing. The primary limitation is that the test is less accurate than other tests for the diagnosis of a current infection. For this reason, recent guidelines have stopped recommending serology tests for diagnosis and instead, have emphasized the use of tests such as stool antigen immunoassays that more accurately confirm current disease.7-10
The Value of Serology Testing
Serology tests for the detection of antibodies to H. pylori are widely available. Most of the FDA-cleared H. pylori serology tests appeared on the market prior to 2006, although there is an additional panel test, which cleared more recently for the detection of antibodies against a variety of pathogens.
Serology testing is based on the host response to H. pylori, which can vary significantly. Test results are dependent on the detection of IgM, IgG, and/or IgA antibodies against the organism. Symptomatic patients also experience inflammation, but serology testing and other noninvasive tests do not measure this response. Most persons who have been exposed to H. pylori will produce antibodies against various cellular components such as membrane fragments, flagellar proteins, and virulence factors, including urease and vacuolating cytotoxin. The antibody responses to these antigens can persist for long periods of time, and in fact, patients can test positive for months or even years after the organism has been eradicated. This persistence of antibodies explains the lower accuracy of serological tests for current H. pylori infections.
Manufacturers take different approaches for the development of serology tests. Tests vary in the class of antibodies they detect. Performance will be affected by the type and purity of the capture antigen immobilized on the membrane. Some experts feel it is important to use local H. pylori strains when developing serology tests. Therefore, some companies produce tests that are based on a mixture of antigens produced from locally isolated strains. Others use pooled antigens from a mixture of geographically distinct strains. Some tests utilize more specific immunogenic proteins such as CagA (cytotoxin associated protein), VacA (vacuolating cytotoxin), and Omp (outer membrane protein) as capture antigens.11 There is some indication that using very specific capture antigens (e.g., vacuolating toxin or the protein encoded by the cagA gene) provides higher correlation with active infections.12
Patients infected with H. pylori will initially develop an IgM response subsequently followed by IgG and IgA antibodies against the organism. Some serology tests will detect primarily IgG, others will target IgA, and some will target all three subclasses. There is no indication that the detection of specific classes of antibodies results in clinically meaningful predictions. Although the Houston Consensus Conference did not support serological assays for the diagnosis of a current H. pylori infection, the conference did note that IgG antibodies against H. pylori were more reliable than the detection of IgM or IgA antibodies.
Test-to-test variation has been reported in several studies. One study compared the performance of four serological assays that differed according to immunoglobulin isotype detected and coating antigen. A stool antigen test was used as the comparator test. The results showed that the performance of the tests varied based on age, health, gender, and ethnicity of the population being tested.13 Because of these differences, reliable cutoffs must be validated with each commercial test to establish performance, identify test-to-test variation, and identify differences in lot-to-lot quality.
Serology testing is the least specific of the commonly used testing methodologies. The higher specificity of the urea breath and fecal antigen tests result in higher negative and positive predictive values than those seen with serology testing. Even so, a recent analysis suggests that serology testing may serve as a screening test. In the analysis, which involved >2,500 patients, several diagnostic approaches were examined: (i) serology for IgG detection, (ii) stool antigen testing, and (iii) an algorithm approach using serum IgG followed by stool antigen testing.14 Serology testing exhibited higher sensitivity but lower specificity, which was not surprising. However, pairing of serology with stool antigen testing provided higher diagnostic accuracy, although this approach required multiple tests.
Because guidelines recommend that serological tests be avoided for diagnosing current infections or when demonstrating eradication of H. pylori following treatment, some major health insurers (e.g., United Healthcare Community Plan, Cigna Medical, Anthem Blue Cross, and Blue Shield) no longer cover serology tests, and some reference laboratories (e.g., Quest Diagnostics, Mayo Clinic) have stopped offering the test.
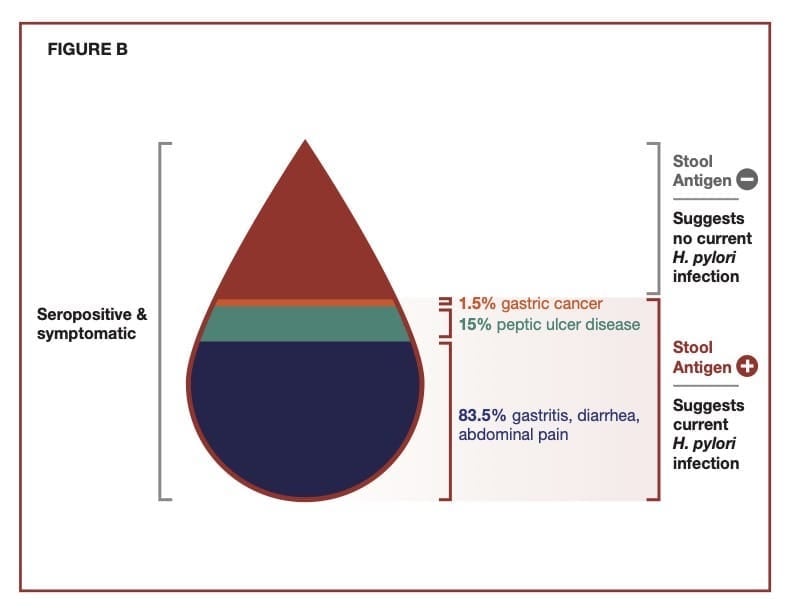
Detecting Current Infections
Stool antigen tests use mixtures of monoclonal or polyclonal antibodies to detect H. pylori-specific antigens in stool samples. In general, the tests perform with sensitivity and specificity values approaching 90% or higher. Importantly, stool antigen tests provide a broader analysis by assessing the overall stomach contents, unlike invasive tests that are based on the analysis of small biopsy specimens.
Commercially available H. pylori stool antigen tests include rapid tests and 96-well ELISA formats. These tests can be performed with unfrozen or frozen specimens and were cleared by comparator studies against biopsy specimens followed by urease and histology, which is considered “gold standard” testing. The rapid tests offer the advantage of being easy to perform with rapid turn-around times, and they do not require specialized equipment. The 96-well test formats are standard ELISA microplate assays that use incubation and washing steps, followed by the addition of substrate for colorimetric determination. The 96-well assays are advantageous when testing large numbers of specimens. All the tests are qualitative.
Clinical evaluations have been performed to demonstrate that the sensitivity and specificity of the tests are comparable to gold standard tests that include endoscopy, histology, and urease testing. The sensitivity and specificity of these stool antigen tests approach 95% with the microwell and rapid formats.15,16 Samples can be stored for several days at 2-8°C and, if not tested in that time period, the samples should be frozen. Samples stored at room temperature or samples in transport media can be used with some of the tests.
Proton pump inhibitors, antibiotics, and bismuth compounds inhibit the growth of H. pylori. Consequently, these medications may cause false negative results if used in the weeks prior to testing for stool antigen or if performing the breath test. Although a positive stool antigen result in patients taking these medications is considered accurate, if a false negative test result is suspected, patients should stop taking the medication for two weeks prior to stool antigen testing. Additional testing should be done after this period. Patients undergoing UBT are required to discontinue use of these medications before testing in all cases.
In general, when comparing the three types of noninvasive tests, stool antigen tests are similar to the urea breath test in performance, but are less expensive, and do not require specialized instrumentation or disposal of radioactive materials. When compared to serology, stool antigen tests are more accurate for detecting current infections. Stool samples from persons with H. pylori infections will vary in consistency and will include solid, semi-solid, and diarrhea stools. Some may have blood and mucus. Stool antigen tests must be robust enough to work with all types of stool specimens.
A primary purpose for diagnostic testing for H. pylori infections is to prevent gastric cancer. A recent study suggests that screening with a breath test or stool antigen test and treating H. pylori infections in families with a history of gastric cancer results in a decrease in both the incidence and mortality from gastric cancer. Screening with a breath test or stool antigen test not only results in a lower incidence, it also provides a more effective cost-basis compared to no screening.17
Establishing a ‘Test-Treat-Test’ Strategy
Patients at-risk of a current H. pylori infection should be tested with assays such as stool antigen tests or breath tests that detect current infections. If a serology test is used, the physician should be aware that a positive test result does not confirm a current infection and should instead reflex to a stool antigen or perform the breath test. A ”test-treat-test” strategy is now broadly adopted in patients with H. pylori infections, and should be used for all patients who are positive upon initial testing.18 Patients treated for H. pylori should undergo posttreatment confirmation of eradication of H. pylori using tests for stool antigen or performing the breath test. After eradication of the organism has been confirmed, no additional testing is needed.
About the Authors
Jodie Y. Lee, M.S., M.B.A., is the director of marketing for TECHLAB, Inc. She has spent 16 years in the life science and diagnostic industries.
David M. Lyerly, Ph.D., is a co-Founder and chief science officer for TECHLAB, Inc. TECHLAB, Inc. manufactures a variety of in vitro diagnostic tests for enteric diseases.
References
1. Shah S, Iyer P, Moss S. AGA Clinical Practice Update on the Management of Refractory Helicobacter pylori Infection: Expert Review. Gastroenterology 2021;160:1831–1841. doi: 10.1053/j.gastro.2020.11.05
2. Katelaris P, Hunt R, Bazzoli F, et al. World Gastroenterology Organisation Global Guidelines Helicobacter pylori May 2021. doi: https://www.worldgastroenterology.org/guidelines/global-guidelines/helicobacter-pylori
3. El-Serag H, Kao J, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992-1002.e6. doi: 10.1016/j.cgh.2018.03.013
4. Helicobacter pylori Diagnosis and Treatment Guidelines. Amer J Managed Care. Copyright © 2021 by Managed Care & Healthcare Communications, LLC
5. Lee J, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med 2015 Jan;3:10. doi: 10.3978/j.issn.2305-5839.2014.11.03
6. Uotani T, Graham D. Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 2015 Jan;3:9. doi: 10.3978/j.issn.2305-5839.2014.12.04
7. Howden C, Blume S, de Lissovoy G. Practice patterns for managing Helicobacter pylori infection and upper gastrointestinal symptoms. Am J Manag Care 2007;13:37-44.
8. Theel, E. Helicobacter pylori infection: serologic testing not recommended. Communique Archive Jan 15, 2016.
9. Murakami T, Scranton R, Brown H, et al. Management of Helicobacter pylori in the United States: results from a national survey of gastroenterology physicians. Prev Med 2017;100:216-222. doi: 10.1016/j.ypmed.2017.04.021
10. Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. BioMed Res Internatl 2016. http://dx.doi.org/10.1155/2016/4819423
11. Roesler B, Rabelo-Goncalves E, Zeitune J. 2014. Virulence factors of Helicobacter pylori: a review. Clin Med Insights: Gastroenterology 2014;7:9-17. doi:10.4137/CGast.S13760
12. Butt J, Blot W, Shrubsole M, et al. Perform of multiplex serology in discriminating active vs past Helicobacter pylori infection in a primarily African American population in the southeastern United States. Helicobacter 2020;25, e12671. doi: 10.1111/hel.12671
13. Biranjia-Hurdoyal S, Seetulsingh-Goorah S. Performances of four Helicobacter pylori serological detection kits using stool antigen test as gold standard. PLoS One 11:e0163834. doi: 10.1371/journal.pone.0163834
14. Bosch D, Krumm N, Wener M, et al. Serology is more sensitive than urea breath test or stool antigen for the initial diagnosis of Helicobacter pylori gastritis when compared with histopathology. Am J Clin Pathol 2020;154:355-365. doi: 10.1093/ajcp/aqaa043
15. Veijola L, Myllyluoma E, Korpela R, et al. Stool antigen tests in the diagnosis of Helicobacter pylori infection before and after eradication therapy. World J Gastroenterol 2005;11:7340-4.
16. Chey W, Wong B. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808-25.
17. Rustgi S, Oh A, Hur C. Testing and treating Helicobacter pylori infection in individuals with family history of gastric cancer is cost-effective. Gastroenterology 2021;161:2051-2052. doi: 10.1053/j.gastro.2021.08.042
18. Chey W, Leontiadis G, Howden C, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Amer J Gastroenterol Vol 112:Feb 2017. doi: 10.1038/ajg.2016.563





