August 3, 2012 report
Water desalination system works up to several times faster than others
(Phys.org) -- As the world population increases and fresh water sources become scarcer, many people will likely rely on technologies that convert salt water to fresh water to meet their most basic needs. Currently, the most common method of water desalination is reverse osmosis, a process that removes water molecules from salt water, leaving salt ions (sodium and chlorine) in the leftover brine. But an alternative method called capacitive desalination (CD), also known as capacitive deionization (CDI), has the potential to operate with greater energy efficiency, lower pressures, and no membrane components compared to reverse osmosis.
Now in a new study, researchers from Stanford University and Lawrence Livermore National Laboratory (LLNL), both located near San Francisco, California, US, have redesigned the architecture of the typical CD system and demonstrated a desalination rate that is 4-10 times faster and a salt concentration reduction that is 3-4 times higher per charge compared with typical CD systems. Their paper is accepted for publication in an upcoming issue of Energy & Environmental Science.
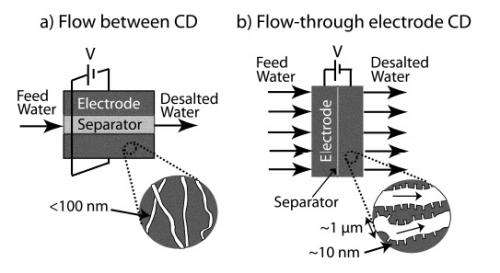
Flow between
CD systems are most often designed so that the salty feed water flows between two porous electrodes, which the researchers call a “flow-between” CD system. By applying a voltage between the electrodes, ions in the feed water are removed and stored in the electric double layers (EDLs) in the micropores of the electrodes. Since opposite charges attract, the negatively charged chlorine anions are electrically adsorbed by the positively charged anode, while the positively charged sodium cations are pulled to the negatively charged cathode. When the electrodes become nearly full, water is pumped out of the system. New feed water is then introduced, and removing the voltage releases ions into a brine stream. These ions are then flushed out of the system and a new cycle can begin.
Despite its potential advantages, flow-between CD systems also have some significant limitations. For one, it takes multiple charging steps to desalinate brackish (moderately salty) water, since these systems typically reduce the concentration of salt water by less than 20 millimolar (mM) per charge. Considering that brackish water has a salinity which can be as high as 500 mM – whereas seawater has a salinity of 500 mM or more – getting the salinity of even mildly brackish water down to a potable water ion concentration of 15 mM or so could take several charging steps. Further, since each desalination step can take 10 times longer than the system charging time, the total desalination time can be very long.
Flow through
Here, the researchers show that they can improve on both these counts by redesigning the CD architecture. Instead of having the feed water flow between electrodes, their alternative structure feeds the water directly through the electrode pores. They call this design a “flow-through electrode” CD system. Although this design was investigated back in the ‘70s, the electrode material and its small pore size resulted in low performance, and there has been little research since then.
As the scientists explain in this study, flow-between CD systems often use traditional carbon aerogels as the electrode material. But here, for the first time in a CD cell, the scientists chose a new electrode material called hierarchical carbon aerogel monoliths (HCAM). They explain that HCAM is better suited for the unique requirements of flow-through electrode systems due to its micron-scale pore network, into which size-tunable nanopores can be etched. As a result, the larger pores provide low hydraulic and electrical resistance since water molecules and ions can easily flow through, while the smaller pores provide a high specific capacitance since they can represent a very large amount of surface area and therefore store large amounts of salt ions.
Besides the electrode material, the second advantage of this architecture is that it can have a much smaller distance between electrodes, called the separator, because this space doesn’t double as the flow channel as in the traditional architecture. By decreasing the separator to a thickness of only 1% of the electrode thickness – whereas the traditional “flow-between” mode commonly uses a 1-to-1 ratio of separator to electrode – the researchers were able to minimize the adverse effects of a thick separator on cell volume and ionic resistance, which otherwise decreases overall desalination performance.
Faster and with fewer cycles
After theoretically showing that a flow-through electrode CD system using HCAM electrodes and a thin separator can compare favorably to the traditional flow-between CD systems in theory, the researchers verified these predictions by fabricating and testing an actual system.
The researchers tested the flow-through electrode CD system with water of salinity between 50 and 250 mM and under applied voltages between 0.75 and 1.5 V. They found that, for water with a salinity of 250 mM at 1.25 V, the system could remove salt at an average rate of 0.96 mg of NaCl per gram of electrode per minute, which is 4-10 times higher than the rates of 0.1-0.25 for flow-between CD systems. They expect this sorption rate could be further improved by charging the system for shorter times, which would allow for more cycles per hour.
Also, whereas flow-between CD systems can only reduce the salt concentration by 20 mM per charge, the new system demonstrated a concentration reduction of up to 70 mM per charge. Both performance benefits could lead to desalinating water more quickly and cheaply than standard CD techniques.
“The high sorption rate increases water throughput, while the high concentration reduction per cycle reduces the number of stages required to desalinate a salt stream,” lead author Matthew Suss at Stanford and LLNL, on behalf of all the researchers, told Phys.org. “This translates into a reduced infrastructure cost for the electrode system. The flow-through geometry also has higher energy efficiency than the traditional flow-between geometry, as it ensures that all of the desalinated water in the system is used. In the previous systems, only the desalted water in the separator was used, but not that in the electrodes, and so the energy used to desalt the water in the electrode pores was largely wasted. Finally, the reduced spacer size reduces the overall resistance of the device, which further decreases energy cost.”
Future of CD
As one of the senior authors, Juan Santiago of Stanford, explained, these improvements make flow-through CD an attractive technique for desalting water, particularly brackish water.
“While CD should theoretically be able to desalinate any water with an efficiency comparable to RO, in its current state, CD is most useful and best applied to the important problem of processing brackish water type salinity solutions at slow rates,” Santiago said. “However, we should not underestimate the importance of treating brackish water. For example, in large regions of the world (including North America), brackish water is projected to be the main source of water for desalination processes that provide drinking water. Brackish water is also the concentration range of effluent of some industrial processes, such as coal bed methane production; treating this water is essential for proper practical waste reduction.”
The researchers added that CD systems could make an impact on desalinating salt water on a small scale, such as in the form of truck-bed sized devices for disaster relief.
“The efficiency of energy recovery devices for RO scales with their size,” Santiago said. “Thus, optimum efficiency is achieved for large plants, but the efficiency decreases significantly for smaller devices. As you reduce the required scale of the desalination plant, CD becomes more competitive and eventually more attractive than RO.”
In the future, the researchers hope to improve upon another advantage of the flow-through system, which is its ability to operate for multiple stages.
“While multiple-stage CD desalination is possible with flow-between, it is usually much harder to do than single-stage, and thus CD has been mostly confined to salinities that can be desalted in a single stage,” said Michael Stadermann at LLNL, one of the senior authors. “We think that flow-through-CD should be much easier to stage due to easier separation of desalination. A next step would be to build a multi-stage pilot system that can desalinate water streams with higher salinity and demonstrate desalination of sea water with good energy efficiency. In the longer term, we plan to scale up and reduce the cost of material synthesis and, eventually, we hope to enable commercialization of this technology.”
Dr. Maarten Biesheuvel of Wetsus, the Netherlands’ Centre of Excellence for Sustainable Water Technology, who also researches CDI systems but was not involved in the current study, thinks that the new flow-through electrode system looks promising for the future of water desalination.
“The achievement of Suss, et al., is a very significant one, opening the eyes of the desalination community to a very different operational mode for CD, with some very interesting and beneficial features,” Biesheuvel said. “The flow-between mode of operation has become the ‘default setting’ for a CD design, admittedly also in our own work. The work of LLNL/Stanford makes us reevaluate if perhaps we have underestimated the potential of flow-through flow to speed up the CD process from the present, slower, diffusion time scale to the faster RC charging time scale, as Suss, et al., have shown is feasible.
“In any case, it is exciting to see how in about five years time the interest in the CD technology has exploded around the world, having established itself as the primary runner-up to the established technologies of reverse osmosis and electrodialysis. Within the US, development activities are now being undertaken at several universities (Stanford, MIT, Drexel, GeorgiaTech) and national laboratories (LLNL, Oak Ridge), and various industrial companies are pursuing commercialization around the world.”
Biesheuvel’s research group will publish a paper in Energy & Environmental Science in which they demonstrate that constant-current operation has advantages over the traditional mode of constant-voltage operation, notably that the salinity of the produced fresh water remains constant in time instead of gradually varying.
More information: Matthew E. Suss, et al. “Capacative desalination with flow-through electrodes.” Energy & Environmental Science. To be published. DOI: 10.1039/c2ee21498a
R. Zhao, et al. “Energy consumption and constant current operation in membrane capacitive deionization.” Energy & Environmental Science. To be published. DOI: 10.1039/c2ee21737f
© 2012 Phys.org