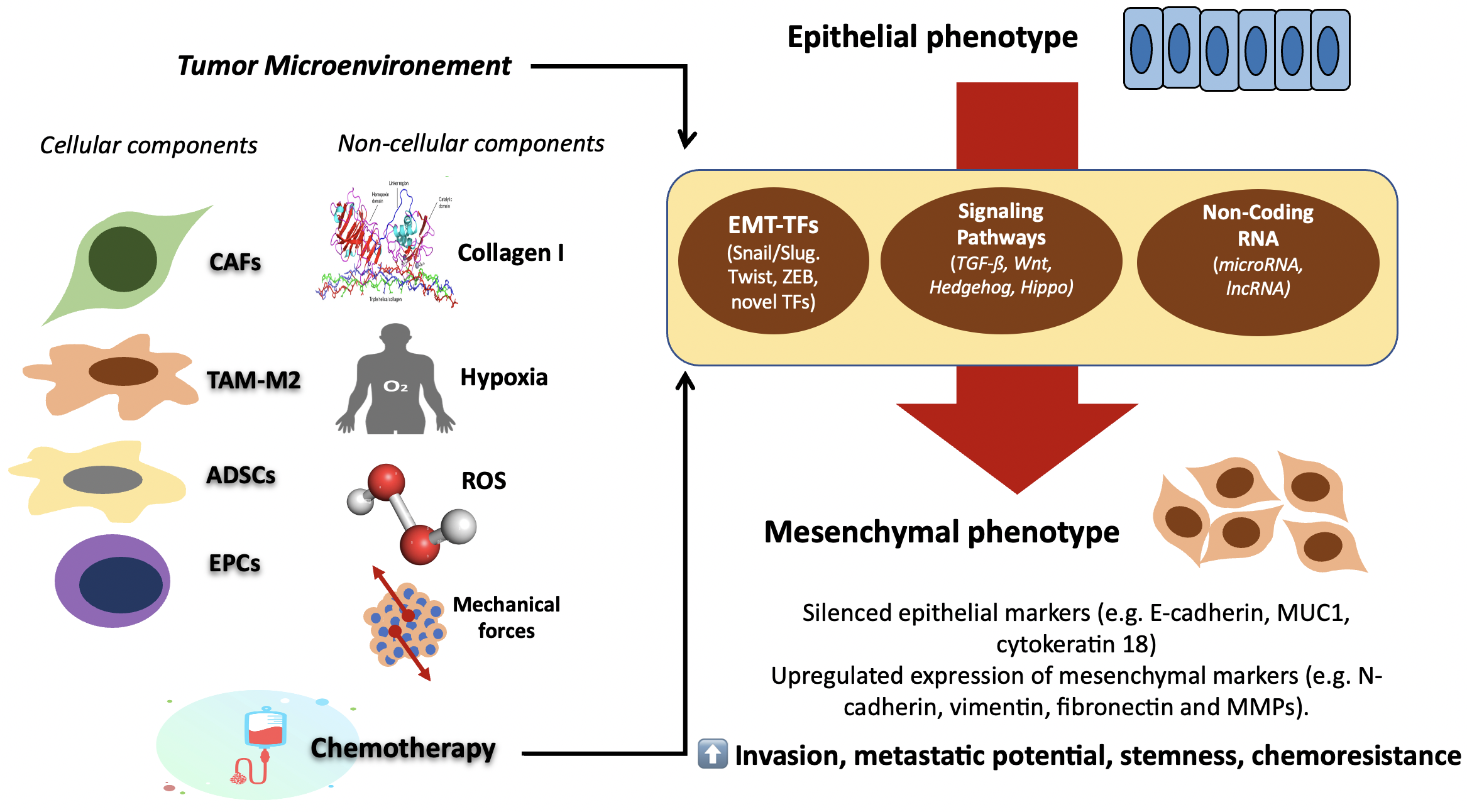
Objective: To understand the basic mechanism and dynamic regulation
that underlies the epithelial-to-mesenchymal transition (EMT) in ovarian cancer
(OC) cells. Mechanism: A literature review using evidences from several
data bases (i.e., PubMed, EMBASE, Web of Science, Medline, Cochrane, Science
Direct, and Google Scholar) were conducted to describe the basic mechanism and
dynamic regulation of EMT in OC cells. Finding in Brief: EMT is a
complex epigenetic reprogramming orchestrated by specific transcription factors
(TFs) and multiple upstream activators and regulators, such as transforming
growth factor-β (TGF-β), Wnt, Hedgehog, and Hippo signaling
pathways. The net result of this cellular reprogramming is the acquisition of
mesenchymal phenotypes with increased invasive and metastatic potential, stemness
properties and chemoresistance. Recent studies have demonstrated that EMT
activation is the result of dynamic and reciprocal interplay between OC cells and
their tumor microenvironment (TME). Cellular or non-cellular component of TME,
external factors related to TME such as hypoxia, oxidative stress, mechanical
forces, as well as exposure to chemotherapy, all play significant role to EMT
induction. Current understanding behind the mechanism of EMT induction in cancer
cells have proposed the idea that EMT is not merely a binary process involving a
complete conversion from epithelial to mesenchymal state, but rather a dynamic
process that encompasses a range of hybrid states, a phenotype that has been
referred to as “partial EMT”. Cells with partial EMT have been known to be more
apoptosis-resistant and have more tumor-initiating potential as compared to those
with complete EMT. Conclusions: Understanding the complex regulatory
network that underlies EMT in OC cells is crucial in order to gain insight in
developing novel and effective treatment strategies for OC.